Vitreo Retinal Surgery Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Vitreo Retinal Surgery Devices Pipeline Market Overview
Vitreo Retinal Surgical Devices are used for surgical procedures that treat eye problems involving the retina, macula, and vitreous fluid. The Vitreo Retinal Surgery Devices pipeline market research report provides comprehensive information about the pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Territories | · The US
· China · Europe · Singapore · Japan · Others |
| Key Regulatory Paths | · 510(k)
· NMPA · CE · HSA · Shonin · ICAC |
| Leading Companies | · AcuSurgical
· Alcon Inc · Bausch & Lomb Inc · Beaver-Visitec International Inc · Boston College · Cambridge Polymer Group Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Vitreo Retinal Surgery Devices Pipeline Market Segmentation by Territories
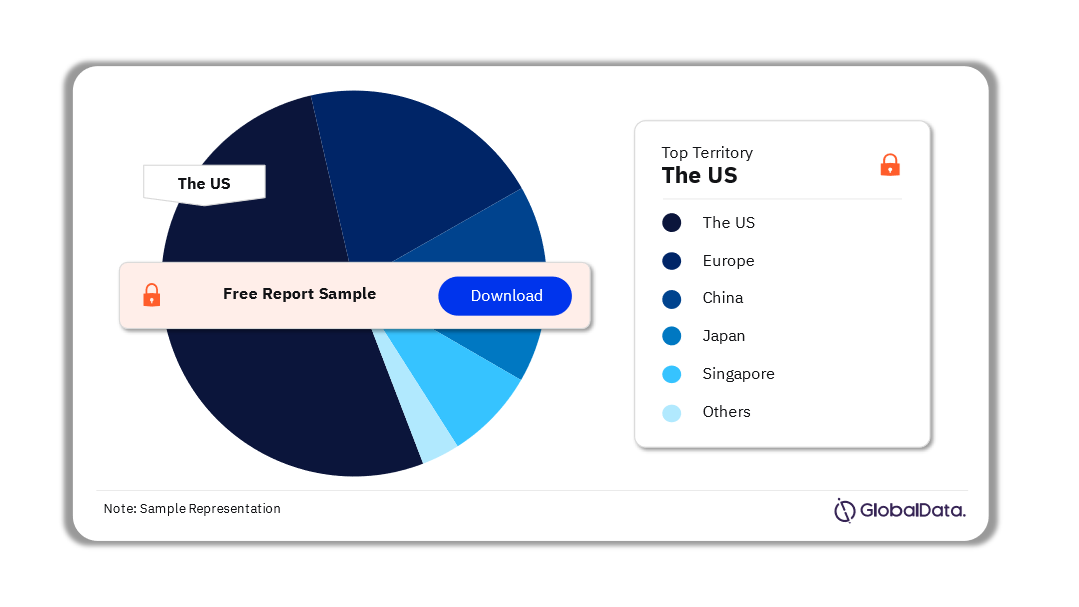
Some of the key territories in the Vitreo Retinal Surgery Devices market are the US, China, Europe, Singapore, Japan, and South Korea among others. As of August 2023, the US accounts for the highest number of Vitreo Retinal Surgery Devices pipeline products.
Vitreo Retinal Surgery Devices Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into The Vitreo Retinal Surgery Devices Pipeline Market, Download a Free Report Sample
Vitreo Retinal Surgery Devices Pipeline Market Segmentation by Regulatory Paths
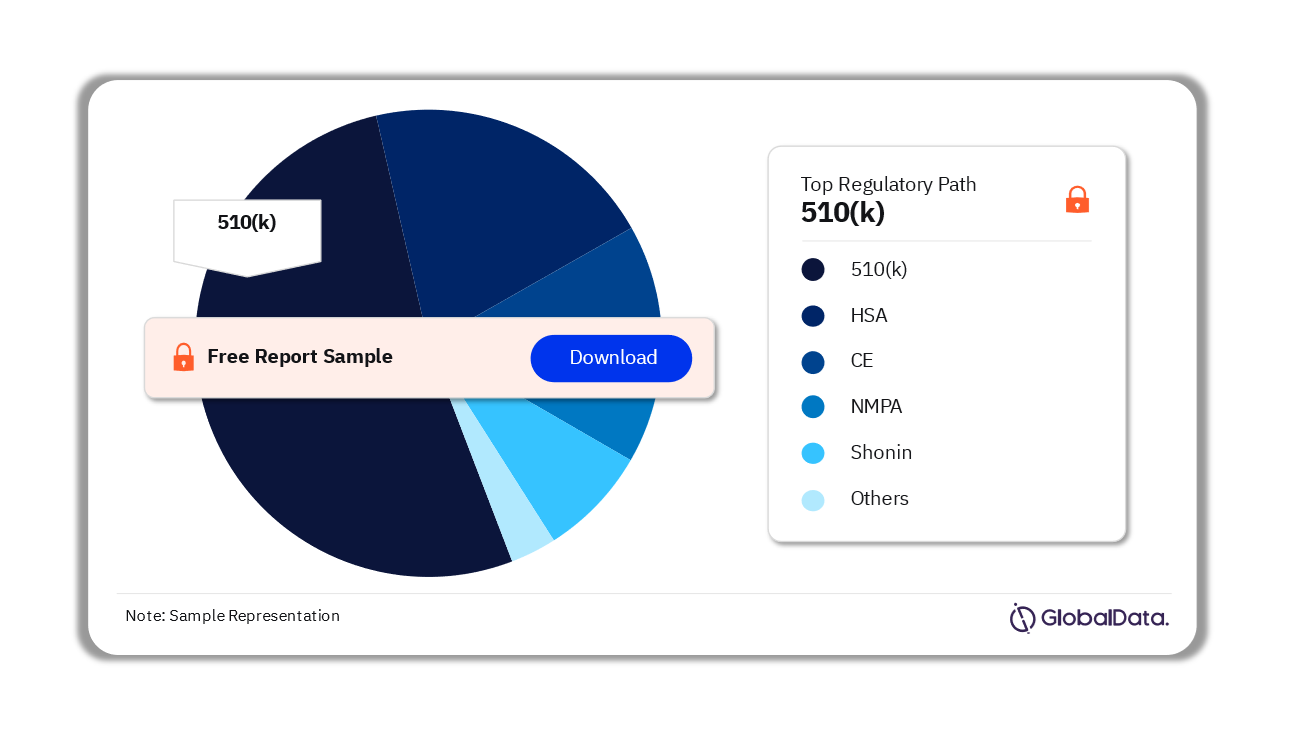
Some of the key regulatory paths in the Vitreo Retinal Surgery Devices pipeline market are 510(k), NMPA, CE, HSA, Shonin, and ICAC among others. As of August 2023, 510(k) is the most followed pathway for pipeline products.
Vitreo Retinal Surgery Devices Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into The Vitreo Retinal Surgery Devices Pipeline Market, Download a Free Report Sample
Vitreo Retinal Surgery Devices Pipeline Market – Competitive Landscape
Some of the key companies in the Vitreo Retinal Surgery Devices pipeline market are AcuSurgical, Alcon Inc, Bausch & Lomb Inc, Beaver-Visitec International Inc, Boston College, and Cambridge Polymer Group Inc among others.
Alcon Inc: Alcon Inc (Alcon) is engaged in the development and manufacturing of devices in the field of ophthalmology. The company’s portfolio encompasses contact lenses and surgical products, including implantables, consumables, and surgical equipment. The company’s products are indicated for the treatment of various conditions such as cataracts, glaucoma, retinal diseases, and refractive errors. It conducts clinical trials to evaluate the safety and efficacy of its products for the prevention and cure of blindness and different eye diseases. Alcon’s pipeline products are developed through collaboration with institutions, medical innovators, research advisors, and academic thought leaders. The company has operations in the Americas, Europe, the Middle East and Africa, and Asia Pacific. Alcon is headquartered in Geneva, Switzerland.
Leading Vitreo Retinal Surgery Devices Companies, 2023
Buy Full Report for More Company Insights into The Vitreo Retinal Surgery Devices Pipeline Market, Download a Free Report Sample
Vitreo Retinal Surgery Devices Pipeline Market Segments
Vitreo Retinal Surgery Devices Pipeline Market Territories Outlook
- China
- United States
- Europe
- Singapore
- Japan
- Others
Vitreo Retinal Surgery Devices Pipeline Market Regulatory Paths Outlook
- NMPA
- 510(k)
- CE
- HSA
- Shonin
- ICAC
Scope
This report provides:
- Extensive coverage of the Vitreo Retinal Surgery Devices under development.
- Details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Vitreo Retinal Surgery Devices and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Vitreo Retinal Surgery Devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
AcuSurgical
Alcon Inc
Bausch & Lomb Inc
Beaver-Visitec International Inc
Boston College
Cambridge Polymer Group Inc
Carnegie Mellon University
Duke University
Johns Hopkins University
Medisse BV (Inactive)
RetinaSense
Synergetics USA, Inc. (Inactive)
The Chinese University of Hong Kong
Tupai (Beijing) Medical Technology Co Ltd
University of Kansas
University of Minnesota
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory has the highest number of pipeline products in the Vitreo Retinal Surgery Devices pipeline market?
As of August 2023, the US has the highest number of pipeline products in the Vitreo Retinal Surgery Devices pipeline market.
-
Which is the most followed regulatory pathway in the Vitreo Retinal Surgery Devices pipeline market?
As of August 2023, 510(k) is the most followed regulatory pathway in the Vitreo Retinal Surgery Devices pipeline market.
-
Who are the major players operating in the Vitreo Retinal Surgery Devices pipeline market?
Some of the key companies in the Vitreo Retinal Surgery Devices pipeline market are AcuSurgical, Alcon Inc, Bausch & Lomb Inc, Beaver-Visitec International Inc, Boston College, and Cambridge Polymer Group Inc among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Vitreo Retinal Surgery Devices reports