Vital Sign Monitors – Pipeline Products by Stage of Development 15
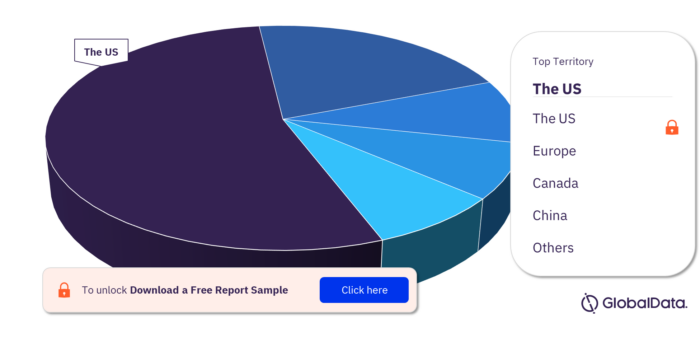
Vital Sign Monitors – Pipeline Products by Territory 16
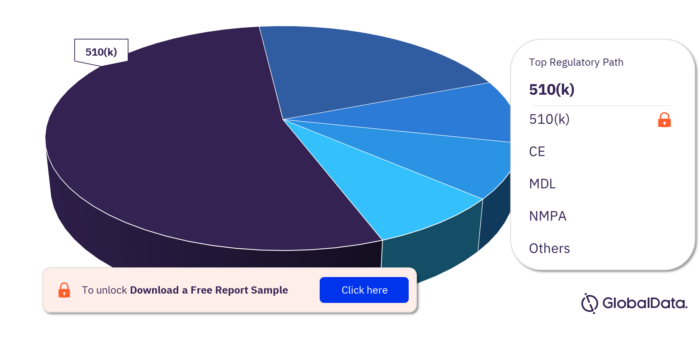
Vital Sign Monitors – Pipeline Products by Regulatory Path 17
Vital Sign Monitors – Pipeline Products by Estimated Approval Date 18
Vital Sign Monitors – Ongoing Clinical Trials 19
Vital Sign Monitors Companies – Pipeline Products by Stage of Development 20
Vital Sign Monitors – Pipeline Products by Stage of Development 24
Aerotel Medical Systems (1988) Ltd Pipeline Products & Ongoing Clinical Trials Overview 27
MDKeeper – Product Status 27
MDKeeper – Product Description 27
Agatsa Software Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 28
SanketLife Multi Vital – Product Status 28
SanketLife Multi Vital – Product Description 28
ARC Devices Ltd. Pipeline Products & Ongoing Clinical Trials Overview 29
MedTemp Connect Vital – Product Status 29
MedTemp Connect Vital – Product Description 29
MedTemp Connect Vital Plus – Product Status 29
MedTemp Connect Vital Plus – Product Description 30
Atoptix LLC Pipeline Products & Ongoing Clinical Trials Overview 31
Atoptix Health Sensor – Product Status 31
Atoptix Health Sensor – Product Description 31
Bertec Corp Pipeline Products & Ongoing Clinical Trials Overview 32
qPCM Device – Product Status 32
qPCM Device – Product Description 32
BioNovus Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 33
OmniSign – Product Status 33
OmniSign – Product Description 33
Biovotion AG Pipeline Products & Ongoing Clinical Trials Overview 34
VSM 2 Tissue Hydration And Sweat – Product Status 34
VSM 2 Tissue Hydration And Sweat – Product Description 34
Bips Health Pipeline Products & Ongoing Clinical Trials Overview 35
BiPS Device – Product Status 35
BiPS Device – Product Description 35
Bips Health – Ongoing Clinical Trials Overview 36
BiPS Device – A Multi-center, Prospective, Pilot Study Intended for Data Collection to Evaluate and Calibrate a Novel Non-invasive Wearable Device for Monitoring Vital Signs 37
Boise State University Pipeline Products & Ongoing Clinical Trials Overview 38
PPG Optical Monitoring And Computing Device – Product Status 38
PPG Optical Monitoring And Computing Device – Product Description 38
CardieX Ltd Pipeline Products & Ongoing Clinical Trials Overview 39
ATCOR Pulse – Product Status 39
ATCOR Pulse – Product Description 40
Vital Sign Monitoring Device – Dementia – Product Status 40
Vital Sign Monitoring Device – Dementia – Product Description 40
CareTaker Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 41
Next Generation Caretaker System – Product Status 41
Next Generation Caretaker System – Product Description 41
VitalStream Platform – Product Status 42
VitalStream Platform – Product Description 42
CareTaker Medical LLC – Ongoing Clinical Trials Overview 43
Next Generation Caretaker System – Validation of a Novel Non-invasive Continuous Blood Pressure Monitor in Children Ages 2- 17 Years-old 44
Celero Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 45
Vitals Monitoring Pill – Product Status 45
Vitals Monitoring Pill – Product Description 45
Celero Systems Inc – Ongoing Clinical Trials Overview 46
Vitals Monitoring Pill – Monitoring Vital Signs with a Wireless Ingestible Device in Subjects Undergoing Polysomnography 47
Cloud DX Inc. Pipeline Products & Ongoing Clinical Trials Overview 48
Pulsewave Health Monitor 2.0 – Product Status 48
Pulsewave Health Monitor 2.0 – Product Description 48
Cortrium ApS Pipeline Products & Ongoing Clinical Trials Overview 49
Cortrium C3 – Oxygen Saturation – Product Status 49
Cortrium C3 – Oxygen Saturation – Product Description 49
CorTronix Biomedical Advancement Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 50
CorCare – Product Status 50
CorCare – Product Description 50
Detectivio AB Pipeline Products & Ongoing Clinical Trials Overview 51
RIA-Device – Product Status 51
RIA-Device – Product Description 51
Empirical Technologies Corporation Pipeline Products & Ongoing Clinical Trials Overview 52
HRWatch – Product Status 52
HRWatch – Product Description 52
Epicore Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 53
Wearable Patch – Product Status 53
Wearable Patch – Product Description 53
Erasmus MC Pipeline Products & Ongoing Clinical Trials Overview 54
Alviscan – Product Status 54
Alviscan – Product Description 54
Fabrixense Pipeline Products & Ongoing Clinical Trials Overview 55
Touchless Unified Fabric Biometric Sensor – Product Status 55
Touchless Unified Fabric Biometric Sensor – Product Description 55
Facedrive Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 56
Bluetooth-Based Wearable Device – Product Status 56
Bluetooth-Based Wearable Device – Product Description 56
TraceSCAN V2 – Product Status 57
TraceSCAN V2 – Product Description 57
FaceHeart Corp Pipeline Products & Ongoing Clinical Trials Overview 58
FaceHeart Vitals – Product Status 58
FaceHeart Vitals – Product Description 58
Fujitsu Ltd Pipeline Products & Ongoing Clinical Trials Overview 59
Wireless Wristband Sensor – Product Status 59
Wireless Wristband Sensor – Product Description 59
G Medical Innovations Ltd Pipeline Products & Ongoing Clinical Trials Overview 60
Prizma Medical Smartphone Jacket – Product Status 60
Prizma Medical Smartphone Jacket – Product Description 60
GE Global Research Pipeline Products & Ongoing Clinical Trials Overview 61
Vital Signs Monitor Wireless Patch – Product Status 61
Vital Signs Monitor Wireless Patch – Product Description 61
GE Healthcare Pipeline Products & Ongoing Clinical Trials Overview 62
Disposable Clinical Monitor – Product Status 62
Disposable Clinical Monitor – Product Description 62
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 63
Non – Contact Vital Sign Detection System – Product Status 63
Non – Contact Vital Sign Detection System – Product Description 63
Hill-Rom Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 64
Vital Signs Patch – Product Status 64
Vital Signs Patch – Product Description 64
Hope College Pipeline Products & Ongoing Clinical Trials Overview 65
Vital Sleep Headband – Product Status 65
Vital Sleep Headband – Product Description 65
Iceni Labs Ltd Pipeline Products & Ongoing Clinical Trials Overview 66
Safescan ApniSense – Product Status 66
Safescan ApniSense – Product Description 66
Safescan RespiSense – Product Status 67
Safescan RespiSense – Product Description 67
Iceni Labs Ltd – Ongoing Clinical Trials Overview 68
Safescan RespiSense – Developing a Non-contact Sleep Apnoea Detector, Suitable for Home Studies – The Safescan Study 69
Impact Instrumentation, Inc. Pipeline Products & Ongoing Clinical Trials Overview 70
LTM/iRevive System – Product Status 70
LTM/iRevive System – Product Description 70
KGPS Israel Ltd Pipeline Products & Ongoing Clinical Trials Overview 71
hereO – Vital Signs Monitor – Product Status 71
hereO – Vital Signs Monitor – Product Description 71
Koronis Biomedical Technologies Corporation Pipeline Products & Ongoing Clinical Trials Overview 72
Point Of Care Neonatal Monitor – Product Status 72
Point Of Care Neonatal Monitor – Product Description 72
Kyushu University Pipeline Products & Ongoing Clinical Trials Overview 73
Portable Microwave Sensor – Product Status 73
Portable Microwave Sensor – Product Description 73
Leman Micro Devices SA Pipeline Products & Ongoing Clinical Trials Overview 74
e-Checkup System – Product Status 74
e-Checkup System – Product Description 74
Lionsgate Technologies Inc. Pipeline Products & Ongoing Clinical Trials Overview 75
Anesthesia Kit – Product Status 75
Anesthesia Kit – Product Description 75
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 76
Wireless Biosensor – Product Status 76
Wireless Biosensor – Product Description 76
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 77
Medical Mirror – Product Status 77
Medical Mirror – Product Description 77
Wearable Vital Signs Monitor – Product Status 78
Wearable Vital Signs Monitor – Product Description 78
Medical Care Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 79
Tele-Health Suite – Heart Vital Signs – Product Status 79
Tele-Health Suite – Heart Vital Signs – Product Description 79
Medieta Oy Pipeline Products & Ongoing Clinical Trials Overview 80
Wearable Wrist Monitor – Product Status 80
Wearable Wrist Monitor – Product Description 80
MS Westfalia GmbH Pipeline Products & Ongoing Clinical Trials Overview 81
Floyd 7000 – Patient monitor – Product Status 81
Floyd 7000 – Patient monitor – Product Description 81
Floyd 8000 – Vital signs monitor – Product Status 82
Floyd 8000 – Vital signs monitor – Product Description 82
Floyd 9000 – Vital signs monitor – Product Status 82
Floyd 9000 – Vital signs monitor – Product Description 83
Floyd 9500 – Patient monitor – Product Status 83
Floyd 9500 – Patient monitor – Product Description 83
Neteera Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 84
Neteera 130H – Product Status 84
Neteera 130H – Product Description 84
Norbert Health Inc Pipeline Products & Ongoing Clinical Trials Overview 85
Ambient Vital Sign Monitor – Product Status 85
Ambient Vital Sign Monitor – Product Description 85
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 86
Sleepi-Band – Product Status 86
Sleepi-Band – Product Description 86
Norwegian University of Science and Technology Pipeline Products & Ongoing Clinical Trials Overview 87
INSTA-Patch – Product Status 87
INSTA-Patch – Product Description 87
Novartis AG Pipeline Products & Ongoing Clinical Trials Overview 88
Digital Mobility Assessment System – Product Status 88
Digital Mobility Assessment System – Product Description 89
Pacific Northwest National Laboratory Pipeline Products & Ongoing Clinical Trials Overview 90
VitalTag – Product Status 90
VitalTag – Product Description 90
PneumoSonics Inc. Pipeline Products & Ongoing Clinical Trials Overview 91
Vital Sign Detection Device – Product Status 91
Vital Sign Detection Device – Product Description 91
Pressao Medical Pipeline Products & Ongoing Clinical Trials Overview 92
BP-ADJUST CV – Product Status 92
BP-ADJUST CV – Product Description 92
Profusa Inc Pipeline Products & Ongoing Clinical Trials Overview 93
Multi-Analyte Sensor – Product Status 93
Multi-Analyte Sensor – Product Description 93
Purdue University Pipeline Products & Ongoing Clinical Trials Overview 94
Skin-Like Electronic Bandage – Product Status 94
Skin-Like Electronic Bandage – Product Description 94
Quanttus Inc Pipeline Products & Ongoing Clinical Trials Overview 95
Vital Sign Data Device – Product Status 95
Vital Sign Data Device – Product Description 95
Quick, LLC Pipeline Products & Ongoing Clinical Trials Overview 96
Vital Sign Monitor – Product Status 96
Vital Sign Monitor – Product Description 96
RDS SAS Pipeline Products & Ongoing Clinical Trials Overview 97
MultiSense Strip – Product Status 97
MultiSense Strip – Product Description 97
RDS SAS – Ongoing Clinical Trials Overview 98
MultiSense Strip – Evaluation of RDS MultiSense in Desaturation Analysis and Effects of Hypoxia on Circulating Oxidative Stress and Mitochondrial Respiration in Healthy Volunteers 99
MultiSense Strip – Study of the Technological and Operational Feasibility of a Remote Automated Monitoring System for the Post-operative Care of Surgical Patients 99
MultiSense Strip – Use of RDS MultiSense in Post-ICU Patients in the COVID-19 Era 99
Recon Health Inc Pipeline Products & Ongoing Clinical Trials Overview 100
Virtual Care Patch – Product Status 100
Virtual Care Patch – Product Description 101
Scanadu Incorporated (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 102
Scanadu Scout – Product Status 102
Scanadu Scout – Product Description 102
Sempulse Pipeline Products & Ongoing Clinical Trials Overview 103
Halo Device – Product Status 103
Halo Device – Product Description 103
Shenzhen Mindray Bio-Medical Electronics Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 104
TrueTymp – Product Status 104
TrueTymp – Product Description 104
Sibel Health Pipeline Products & Ongoing Clinical Trials Overview 105
Anne – Product Status 105
Anne – Product Description 105
Sonica Health Pipeline Products & Ongoing Clinical Trials Overview 106
ADAM – Product Status 106
ADAM – Product Description 106
Sonica Health – Ongoing Clinical Trials Overview 107
ADAM – Open-label, Single Arm, Multi-centre, Prospective First-in-human Study to Assess the Safety of the ADAM System 108
ADAM – Wearable Sensor to Monitor COVID-19 Like Signs and Symptoms 108
Sotera Wireless Inc Pipeline Products & Ongoing Clinical Trials Overview 109
OmniScan – Product Status 109
OmniScan – Product Description 109
Speer Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 110
HemoPod – Product Status 110
HemoPod – Product Description 110
The Chinese University of Hong Kong Pipeline Products & Ongoing Clinical Trials Overview 111
Mobile Wireless Device – Product Status 111
Mobile Wireless Device – Product Description 111
Non-contact Measurement Device – Product Status 112
Non-contact Measurement Device – Product Description 112
Photoplethysmograph Measuring Device – Product Status 112
Photoplethysmograph Measuring Device – Product Description 113
The University of British Columbia Pipeline Products & Ongoing Clinical Trials Overview 114
Vital Signs Monitoring Device – Product Status 114
Vital Signs Monitoring Device – Product Description 114
TruVitals Inc Pipeline Products & Ongoing Clinical Trials Overview 115
VitalOne Device – Product Status 115
VitalOne Device – Product Description 115
University Medical Center Utrecht Pipeline Products & Ongoing Clinical Trials Overview 116
Nightingale Monitoring System – Product Status 116
Nightingale Monitoring System – Product Description 116
University of Aberdeen Pipeline Products & Ongoing Clinical Trials Overview 117
Hand-held Sensor Device – Product Status 117
Hand-held Sensor Device – Product Description 117
University of California Irvine Pipeline Products & Ongoing Clinical Trials Overview 118
Slap Band Vitals Monitor – Product Status 118
Slap Band Vitals Monitor – Product Description 118
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 119
Epidermal Electronic System – Maternal And Fetal Monitoring – Product Status 119
Epidermal Electronic System – Maternal And Fetal Monitoring – Product Description 119
Wearable Ultrasound Patch – Product Status 120
Wearable Ultrasound Patch – Product Description 120
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 121
Wireless Vital Sign Monitor – Product Status 121
Wireless Vital Sign Monitor – Product Description 121
University of Glasgow Pipeline Products & Ongoing Clinical Trials Overview 122
Clinical Sensor – Home Monitoring – Product Status 122
Clinical Sensor – Home Monitoring – Product Description 122
University of Illinois Pipeline Products & Ongoing Clinical Trials Overview 123
Skin-Like Device – Product Status 123
Skin-Like Device – Product Description 123
University of Oxford Pipeline Products & Ongoing Clinical Trials Overview 124
Abnormal Systems Behaviour Monitoring Tool – Product Status 124
Abnormal Systems Behaviour Monitoring Tool – Product Description 124
University of Technology Sydney Pipeline Products & Ongoing Clinical Trials Overview 125
Lachesis Vital Signs Monitor – Product Status 125
Lachesis Vital Signs Monitor – Product Description 125
University of Texas at Austin Pipeline Products & Ongoing Clinical Trials Overview 126
FreePulse – Product Status 126
FreePulse – Product Description 126
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 127
Solar Powered Augmented Lenses – Product Status 127
Solar Powered Augmented Lenses – Product Description 127
University of Waterloo Pipeline Products & Ongoing Clinical Trials Overview 128
Vital Sign Monitoring Device – Product Status 128
Vital Sign Monitoring Device – Product Description 128
Vitalerter Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
VITALERTER Next – Product Status 129
VITALERTER Next – Product Description 129
VitalTracer Ltd Pipeline Products & Ongoing Clinical Trials Overview 130
VT-Patch – Product Status 130
VT-Patch – Product Description 130
VT-Watch – Product Status 131
VT-Watch – Product Description 131
VitalTracer Ltd – Ongoing Clinical Trials Overview 132
VT-Watch – Validation of Vital Signs Recording with VT-Patch or VT-Watch Connected Devices in Children Hospitalized in NICU and PICU 133
VT-Patch – Validation of Continuous Monitoring of Vital Signs in Children with VTPatch Connected Device in the Pediatric Intensive Care Unit 134
VT-Patch – Validation of Vital Signs Recording with VT-Patch or VT-Watch Connected Devices in Children Hospitalized in NICU and PICU 134
VitaScope Pipeline Products & Ongoing Clinical Trials Overview 135
Vital Signs Monitoring System – Product Status 135
Vital Signs Monitoring System – Product Description 135
VivaLnk, Inc. Pipeline Products & Ongoing Clinical Trials Overview 136
Vital Scout – Opiod Overdose – Product Status 136
Vital Scout – Opiod Overdose – Product Description 136
Worcester Polytechnic Institute Pipeline Products & Ongoing Clinical Trials Overview 137
Smartphone Vital Sign Monitor – Product Status 137
Smartphone Vital Sign Monitor – Product Description 137
Glossary 168
![]()