Transcatheter Valve Delivery Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Transcatheter Valve Delivery Devices Pipeline Market Report Overview
Transcatheter Valve Delivery Devices are designed to deliver heart valves using the minimally invasive heart procedures. The Transcatheter Valve Delivery Devices pipeline market research report provides comprehensive information about the Transcatheter valve delivery devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Transcatheter Valve Delivery Devices Pipeline Market Segmentation by Territories
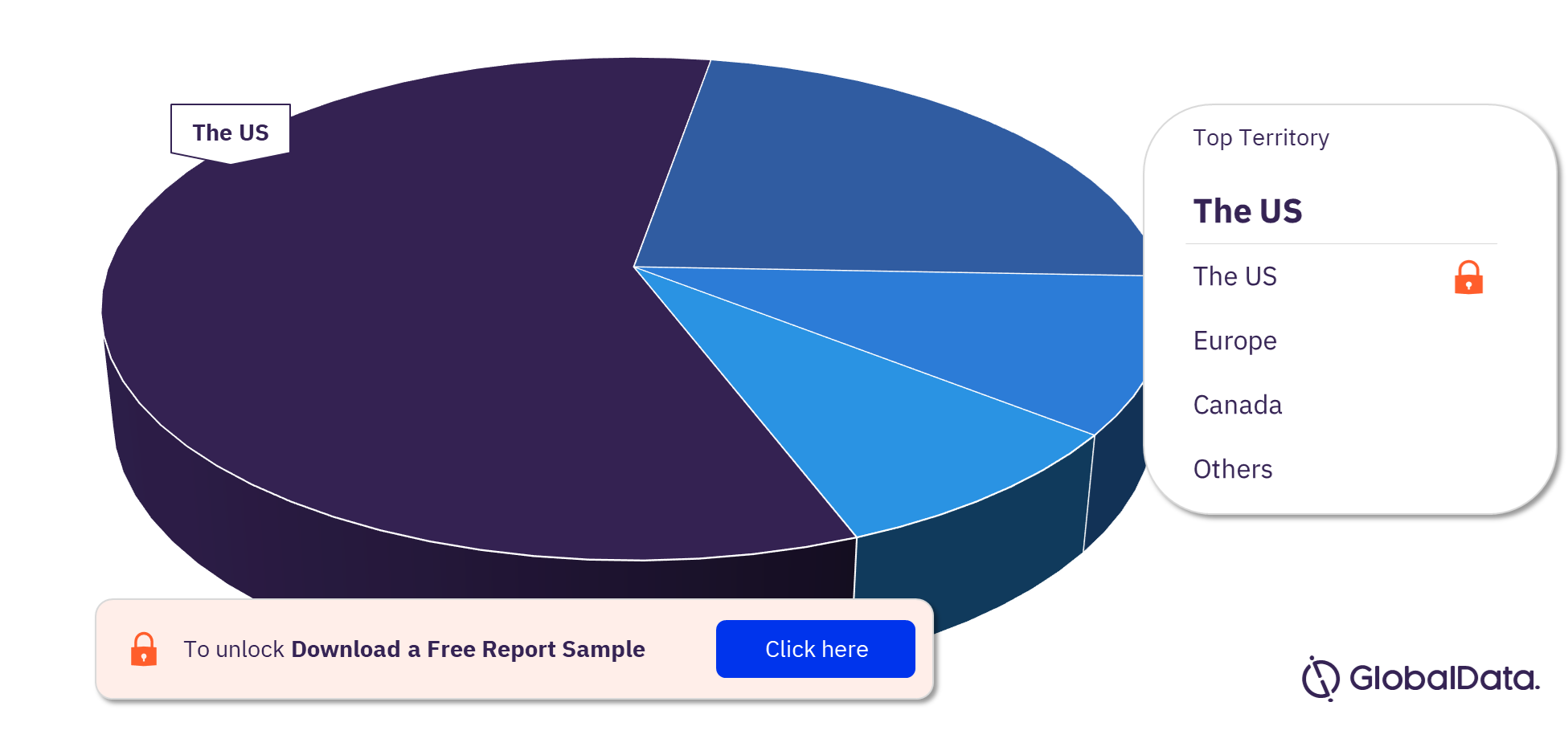
Some of the key territories in the Transcatheter valve delivery devices pipeline market are the US, Europe, and Canada. In 2023, the US has the highest number of pipeline products.
Transcatheter Valve Delivery Devices Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the Transcatheter valve delivery devices pipeline market, download a free report sample
Transcatheter Valve Delivery Devices Pipeline Market Segmentation by Regulatory Paths
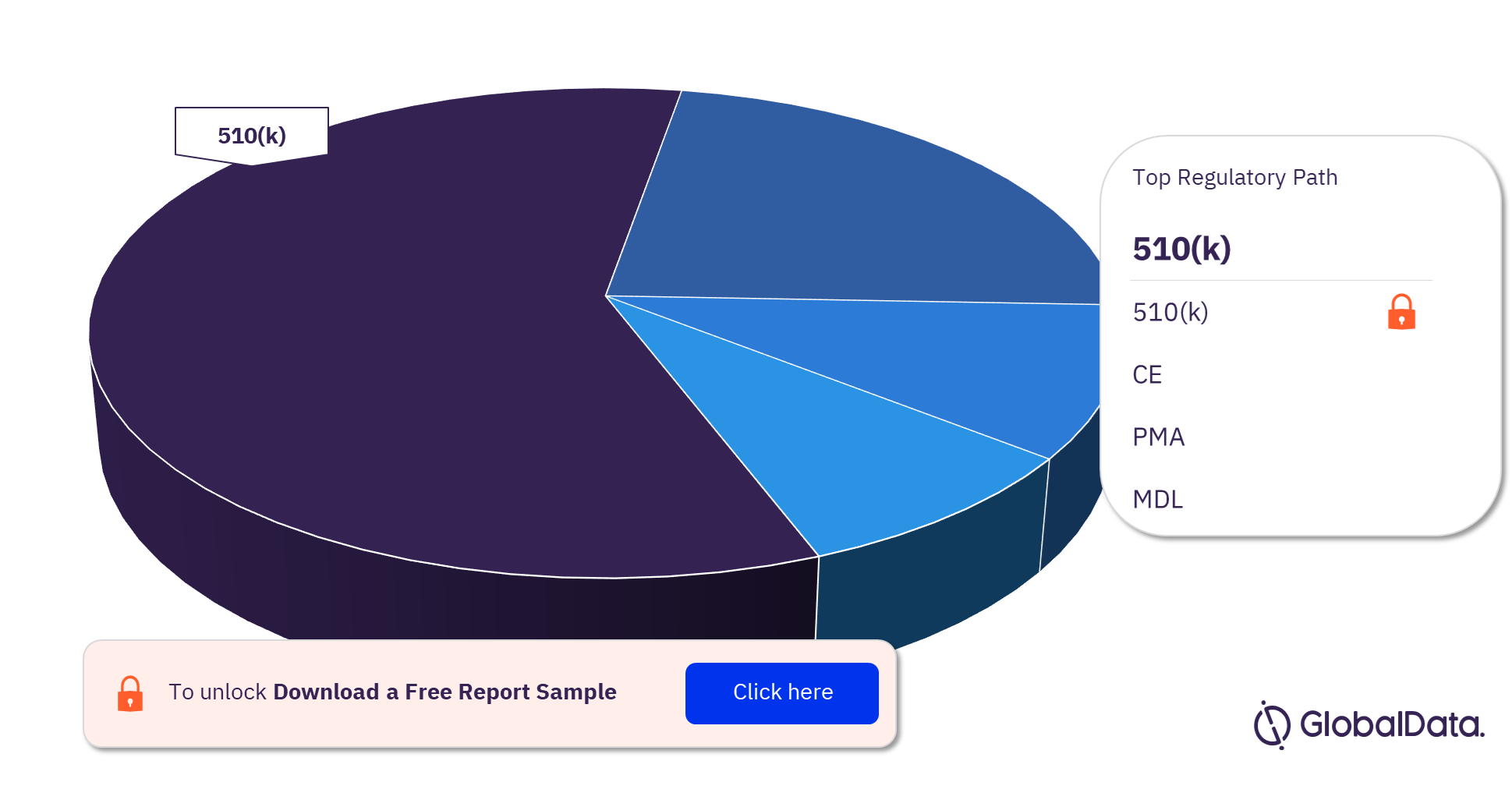
The key regulatory paths in the Transcatheter valve delivery devices pipeline market are 510(k), CE, PMA, and MDL. In 2023, 510(k) was the most followed pathway for Transcatheter valve delivery devices pipeline products.
Transcatheter Valve Delivery Devices Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory paths into the Transcatheter valve delivery devices pipeline market, download a free report sample
Transcatheter Valve Delivery Devices Pipeline Market- Competitive Landscape
Some of the key companies in the Transcatheter valve delivery devices pipeline market are Anteris Technologies Ltd, Colibri Heart Valve LLC, Florida International University, InspireMD Inc, JC Medical, Inc., Meril Life Sciences Pvt Ltd, NaviGate Cardiac Structures, Inc., St. Jude Medical LLC, Symetis SA, and Thoratec LLC.
Anteris Technologies Ltd: Headquartered in Brisbane, Queensland, Australia, Anteris Technologies Ltd, (Anteris Technologies), formerly Admedus Ltd develops, manufactures and distributes medical devices and technologies with focus on tissue engineering and immunotherapies. The company’s product portfolio includes ADAPT technology, a next generation bio scaffold that reengineers xenograft tissue into a pure collagen scaffold and DurAVR heart valve, a 3D single piece aortic valve that creates a wider valve opening and better blood flow.
Colibri Heart Valve LLC: Headquartered in Broomfield, Colorado, the US, Colibri Heart Valve LLC (Colibri Heart Valve) is a privately held medical device company that researches and develops novel, patent protected, and structural heart technologies. The company’s products include Colibri transcatheter aortic valve implantation system, pipeline products, and clinical development products. It also develops a pre-mounted, pre-crimped, pre-loaded, and ready-for-use balloon expandable transcatheter aortic valve implantation system through its proprietary tissue technology and valve design.
Florida International University: Headquartered in Miami, Florida, the US, Florida International University (FIU) operates as a public research university. The university offers degrees in both undergraduate and graduate level curriculum. It also provides degree programs in arts and design, business and economics, education, hospitality management, humanities and culture, medicines and health, public policy, sciences and engineering, and technology. Furthermore, FIU offers support services including disability resource center, career services, counseling and psychological services, student food pantry, student health, TRIO support services, libraries, undergraduate advising, victim empowerment program, wellness and recreation centers and others.
Transcatheter Valve Delivery Devices Pipeline Market Report Overview
| Key Territories | The US, Europe, and Canada |
| Key Regulatory Paths | 510(k), CE, PMA, and MDL |
| Leading Companies | Anteris Technologies Ltd, Colibri Heart Valve LLC, Florida International University, InspireMD Inc, JC Medical, Inc., Meril Life Sciences Pvt Ltd, NaviGate Cardiac Structures, Inc., St. Jude Medical LLC, Symetis SA, and Thoratec LLC |
Segments Covered in the Report
Transcatheter Valve Delivery Devices Pipeline Market Territories Outlook
- The US
- Europe
- Canada
Transcatheter Valve Delivery Devices Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- PMA
- MDL
Scope
- Extensive coverage of the Transcatheter valve delivery devices under development.
- Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates.
- Reviews of major players involved in the pipeline product development.
- Provides key clinical trial data related to ongoing clinical trials such as trial phase, trial status, trial start and end dates, and the number of trials of the major Transcatheter valve delivery devices pipeline products.
- Review of Recent Developments in the segment / industry.
Reasons to Buy
The Transcatheter valve delivery devices report enables you to:
- Access significant competitor information, analysis, and insights to improve your R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of Transcatheter valve delivery devices under development.
- Formulate market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
Colibri Heart Valve LLC
Florida International University
InspireMD Inc
JC Medical, Inc.
Meril Life Sciences Pvt Ltd
NaviGate Cardiac Structures, Inc.
St. Jude Medical LLC
Symetis SA
Thoratec LLC
Thubrikar Aortic Valve, Inc.
TRiCares SAS
University College London
University of California
University of California Irvine
University of Nebraska
University of Pennsylvania
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the Transcatheter valve delivery devices pipeline market?
Some of the key territories in the Transcatheter Valve Delivery Devices pipeline market are the US, Europe, and Canada.
-
What are the key regulatory paths in the Transcatheter valve delivery devices pipeline market?
The key regulatory paths in the Transcatheter Valve Delivery Devices pipeline market are 510(k), CE, PMA, and MDL.
-
What are Transcatheter valve delivery devices used for?
Transcatheter Valve Delivery Devices are used to deliver heart valves using the minimally invasive heart procedures.
-
Which are the leading companies in the Transcatheter valve delivery devices pipeline market?
Some of the leading companies in the Transcatheter valve delivery devices pipeline market are Anteris Technologies Ltd, Colibri Heart Valve LLC, Florida International University, InspireMD Inc, JC Medical, Inc., Meril Life Sciences Pvt Ltd, NaviGate Cardiac Structures, Inc., St. Jude Medical LLC, Symetis SA, and Thoratec LLC.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Transcatheter valve delivery devices reports