Transcatheter Mitral Valve Repair (TMVR) – Pipeline Products by Stage of Development 12
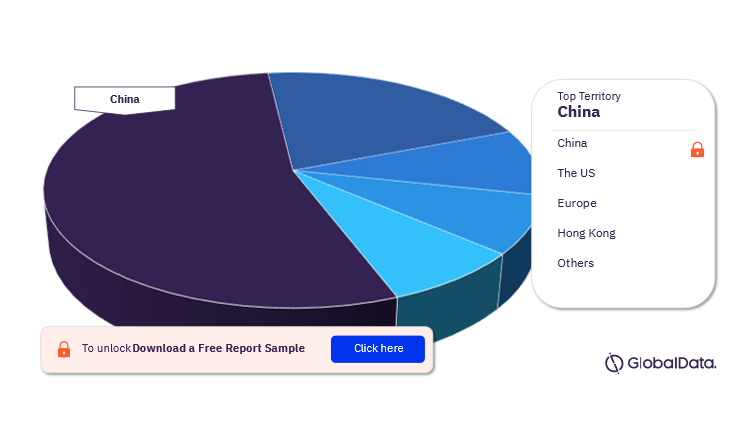
Transcatheter Mitral Valve Repair (TMVR) – Pipeline Products by Territory 13
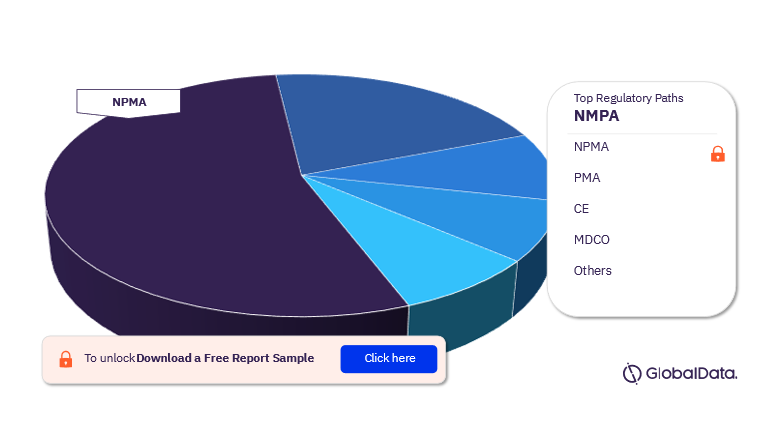
Transcatheter Mitral Valve Repair (TMVR) – Pipeline Products by Regulatory Path 14
Transcatheter Mitral Valve Repair (TMVR) – Pipeline Products by Estimated Approval Date 15
Transcatheter Mitral Valve Repair (TMVR) – Ongoing Clinical Trials 16
Transcatheter Mitral Valve Repair (TMVR) Companies – Pipeline Products by Stage of Development 17
Transcatheter Mitral Valve Repair (TMVR) – Pipeline Products by Stage of Development 19
4C Medical Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 21
Fully Recapturable and Retrievable Transseptal Delivery System – Product Status 21
Fully Recapturable and Retrievable Transseptal Delivery System – Product Description 21
Affluent Medical SASU Pipeline Products & Ongoing Clinical Trials Overview 22
Epygon – Product Status 22
Epygon – Product Description 22
Affluent Medical SASU – Ongoing Clinical Trials Overview 23
Epygon – First-in-human Clinical Study for Treatment of Severe Mitral Valve Insufficiency with Epygon Transcatheter Mitral Valve System (MINERVA FIH) 24
Anchorvalve Pipeline Products & Ongoing Clinical Trials Overview 25
Sutureless Trans-Catheter Mitral Valve Replacement (TMVR) – Product Status 25
Sutureless Trans-Catheter Mitral Valve Replacement (TMVR) – Product Description 25
AVVie GmbH Pipeline Products & Ongoing Clinical Trials Overview 26
Mitral Butterfly – Product Status 26
Mitral Butterfly – Product Description 26
Cardiac Dimensions Inc Pipeline Products & Ongoing Clinical Trials Overview 27
CARILLON Mitral Contour System – Product Status 27
CARILLON Mitral Contour System – Product Description 27
Cardiac Dimensions Inc – Ongoing Clinical Trials Overview 28
CARILLON Mitral Contour System – An Initial Evaluation of the Carillon Mitral Contour System for Treatment of Atrial Function Mitral Regurgitation 29
CARILLON Mitral Contour System – Assessment of the Carillon Mitral Contour System in Treating Heart Failure With at Least Mild Functional Mitral Regurgitation 29
CARILLON Mitral Contour System – European Registry of Transcatheter Repair for Secondary Mitral Regurgitation 29
CARILLON Mitral Contour System – Pivotal Trial for Carillon Mitral Contour System 30
CARILLON Mitral Contour System – The CINCH-FMR Post-Market Registry: Percutaneous Repair in Functional Mitral Regurgitation 30
CardioMech AS Pipeline Products & Ongoing Clinical Trials Overview 31
Transcatheter Mitral Valve Repair System – Product Status 31
Transcatheter Mitral Valve Repair System – Product Description 31
CardioMech AS – Ongoing Clinical Trials Overview 32
Transcatheter Mitral Valve Repair System – Early Feasibility Study of the CardioMech Mitral Valve Repair System (MVRS) 33
coramaze technologies GmbH Pipeline Products & Ongoing Clinical Trials Overview 34
Mitramaze Valve Repair System – Product Status 34
Mitramaze Valve Repair System – Product Description 34
Dura LLC Pipeline Products & Ongoing Clinical Trials Overview 35
Sutra TMVR System – Product Status 35
Sutra TMVR System – Product Description 35
Edwards Lifesciences Corp Pipeline Products & Ongoing Clinical Trials Overview 36
Monarc Transcatheter Mitral Repair System – Product Status 36
Monarc Transcatheter Mitral Repair System – Product Description 36
PASCAL Ace Transcatheter Mitral Valve Repair System – Product Status 37
PASCAL Ace Transcatheter Mitral Valve Repair System – Product Description 37
PASCAL Precision Transcatheter Valve Repair System – Product Status 37
PASCAL Precision Transcatheter Valve Repair System – Product Description 38
PASCAL Transcatheter Mitral Valve Repair System – DMR – Product Status 38
PASCAL Transcatheter Mitral Valve Repair System – DMR – Product Description 38
PASCAL Transcatheter Mitral Valve Repair System – FMR – Product Status 39
PASCAL Transcatheter Mitral Valve Repair System – FMR – Product Description 39
Edwards Lifesciences Corp – Ongoing Clinical Trials Overview 40
PASCAL Transcatheter Mitral Valve Repair System – DMR – Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical Trial (CLASP IID/IIF): A Prospective, Multicenter, Randomized, Controlled Pivotal Trial to Evaluate the Safety and Effectiveness of Transcatheter Mitral Valve Repair with the Edwards PASCAL Transcatheter Valve Repair System Compared to Abbott MitraClip in Patients with Mitral Regurgitation 41
PASCAL Transcatheter Mitral Valve Repair System – DMR – European Registry of Transcatheter Repair for Secondary Mitral Regurgitation 41
PASCAL Transcatheter Mitral Valve Repair System – DMR – The CLASP Study Edwards PASCAL TranScatheter Mitral Valve Repair System Study 41
PASCAL Transcatheter Mitral Valve Repair System – FMR – Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical Trial (CLASP IID/IIF): A Prospective, Multicenter, Randomized, Controlled Pivotal Trial to Evaluate the Safety and Effectiveness of Transcatheter Mitral Valve Repair with the Edwards PASCAL Transcatheter Valve Repair System Compared to Abbott MitraClip in Patients with Mitral Regurgitation 42
PASCAL Transcatheter Mitral Valve Repair System – FMR – Transcatheter Repair of Mitral Regurgitation With Edwards PASCAL Transcatheter Valve Repair System: A European Prospective, Multicenter Post Market Clinical Follow-up (PMCF) 42
Emory University Pipeline Products & Ongoing Clinical Trials Overview 43
MitraPlug – Product Status 43
MitraPlug – Product Description 43
Enlight Medical Technologies (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 44
NovoClasp Transfemoral Mitral Valve Repair System – Product Status 44
NovoClasp Transfemoral Mitral Valve Repair System – Product Description 44
Second-Generation Mitral Valve Repair System – Product Status 45
Second-Generation Mitral Valve Repair System – Product Description 45
Half Moon Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 46
Half Moon TMVr System – Product Status 46
Half Moon TMVr System – Product Description 46
Half Moon Medical Inc – Ongoing Clinical Trials Overview 47
Half Moon TMVr System – Evaluation of the Safety and Performance of the Half Moon Transcatheter Mitral Valve Repair System in High Risk Patients with Severe, Symptomatic Mitral Regurgitation 48
Hangzhou DeJin Medtech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 49
MitralStitch – Product Status 49
MitralStitch – Product Description 49
Hangzhou DeJin Medtech Co Ltd – Ongoing Clinical Trials Overview 50
MitralStitch – A Prospective, Multicenter, Single Group Assignment Study for Evaluating the Safety and Effectiveness of MitralStitch Mitral Valve Repair System in Patients with Moderate to Severe and Severe Mitral Regurgitation 51
MitralStitch – To Evaluate the Effectiveness and Safety of MitralStitch Mitral Valve Repair System – A Clinical Application of New Technology 51
Hangzhou Valgen Medtech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 52
Dragonfly Transcatheter Mitral Valve Clamping System – Product Status 52
Dragonfly Transcatheter Mitral Valve Clamping System – Product Description 52
Hangzhou Valued Medtech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
DragonFly – Product Status 53
DragonFly – Product Description 53
Hangzhou Valued Medtech Co Ltd – Ongoing Clinical Trials Overview 54
DragonFly – A Clinical Study of Management of Severe Tricuspid Regurgitation with Transcatheter Mitral Valve Clamping System 55
DragonFly – A Prospective, Multicenter, Objective Performance Criteria Study to Evaluate the Safety and Effectiveness of Dragonfly Transcatheter Mitral Valve Repair System for the Treatment of Degenerative Mitral Regurgitation (DMR) Subjects 55
DragonFly – A Prospective, Multicenter, Objective Performance Criteria Study to Evaluate the Safety and Effectiveness of Dragonfly Transcatheter Mitral Valve Repair System for the Treatment of Functional Mitral Regurgitation (FMR) Subjects 55
DragonFly – Dragonfly-M Transcatheter Mitral Valve Repair System Early Feasibility Study 56
HighLife SAS Pipeline Products & Ongoing Clinical Trials Overview 57
Peijia HighLife Transcatheter Mitral Valve Replacement – Product Status 57
Peijia HighLife Transcatheter Mitral Valve Replacement – Product Description 57
HighLife SAS – Ongoing Clinical Trials Overview 58
Peijia HighLife Transcatheter Mitral Valve Replacement – A Prospective, Multicentric Clinical Trial Protocol to Evaluate the Safety and Efficacy of the HighLife Trans-Septal Transcatheter Mitral Valve Replacement System for the Treatment of Moderate-severe or Severe Mitral Regurgitation With Single-arm Objective Performance Criteria 59
Peijia HighLife Transcatheter Mitral Valve Replacement – An Early Feasibility Study of the HighLife 28mm Trans-septal Transcatheter Mitral Valve Replacement System 59
Peijia HighLife Transcatheter Mitral Valve Replacement – Feasibility Study of the HighLife 28mm Trans-septal Trans-catheter Mitral Valve in Patients with Moderate-severe or Severe Mitral Regurgitation and at High Surgical Risk 59
Peijia HighLife Transcatheter Mitral Valve Replacement – HighLife Transcatheter Mitral Valve Replacement System for Severe Mitral Regurgitation in Patients at High Surgical Risk 60
Peijia HighLife Transcatheter Mitral Valve Replacement – HighLife TSMVR Feasibility Study of the Open Cell CLARITY Valve in Patients with Moderate-severe or Severe MR, High Surgical Risk and with a High Risk for Left Ventricular Outflow Tract Obstruction (LVOTO) 60
Peijia HighLife Transcatheter Mitral Valve Replacement – Study to Evaluate the Efficacy of HighLife Trans-septal Mitral Valve Replacement System in Patients with Mitral Valve Disease 60
InnovHeart SRL Pipeline Products & Ongoing Clinical Trials Overview 61
Saturn TMVR Prosthesis – Transapical – Product Status 61
Saturn TMVR Prosthesis – Transapical – Product Description 62
Saturn TMVR Prosthesis – Transeptal – Product Status 62
Saturn TMVR Prosthesis – Transeptal – Product Description 63
Saturn TMVR Prosthesis – Transfemoral – Product Status 63
Saturn TMVR Prosthesis – Transfemoral – Product Description 64
InnovHeart SRL – Ongoing Clinical Trials Overview 65
Saturn TMVR Prosthesis – Transapical – SATURN Transcatheter Mitral Valve Replacement for Functional Mitral Regurgitation 66
Jenscare Scientific Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 67
JensClip – Product Status 67
JensClip – Product Description 67
MitraPatch – Product Status 68
MitraPatch – Product Description 68
Jenscare Scientific Co Ltd – Ongoing Clinical Trials Overview 69
JensClip – A Prospective, Multicenter, Single-group Target Value Clinical Study to Evaluate the Safety and Efficacy of JensClip Transcatheter Valve Repair System in the Treatment of Moderate to Severe Degenerative Mitral Regurgitation 70
JensClip – Clinical Study Protocol of Jensclip Transcatheter Valve Clip and Delivery System for the Treatment of High-risk Patients with Moderate to Severe or Severe Mitral Regurgitation 70
Jiangsu Zhenyi Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 71
Neonova Transcatheter Mitral Valve Repair System – Product Status 71
Neonova Transcatheter Mitral Valve Repair System – Product Description 71
Kekai Life Sciences Pipeline Products & Ongoing Clinical Trials Overview 72
Lifeclip – Product Status 72
Lifeclip – Product Description 72
Lepu Medical Technology (Beijing) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 73
Transcatheter Annulus Repair System – Product Status 73
Transcatheter Annulus Repair System – Product Description 73
Medfree Inc Pipeline Products & Ongoing Clinical Trials Overview 74
MEDFREE Valve Repair System – Product Status 74
MEDFREE Valve Repair System – Product Description 74
Medtentia International Ltd Oy Pipeline Products & Ongoing Clinical Trials Overview 75
CathHELIX – Product Status 75
CathHELIX – Product Description 75
Micro Interventional Devices, Inc. Pipeline Products & Ongoing Clinical Trials Overview 76
Permavalve – Product Status 76
Permavalve – Product Description 76
MicroPort CardioFlow Medtech Corp Pipeline Products & Ongoing Clinical Trials Overview 77
Mitral Valve Repair Device – Product Status 77
Mitral Valve Repair Device – Product Description 77
Mitral Heal Pipeline Products & Ongoing Clinical Trials Overview 78
Off-Pump Transapical Mitral Valve Device – Product Status 78
Off-Pump Transapical Mitral Valve Device – Product Description 78
Mitria Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 79
Transcatheter Mitral Valve Repair Device – Product Status 79
Transcatheter Mitral Valve Repair Device – Product Description 79
Neochord Inc Pipeline Products & Ongoing Clinical Trials Overview 80
NeoChord DS1000 – Product Status 80
NeoChord DS1000 – Product Description 80
NeXuS Transcatheter Mitral Chordal Repair Device – Product Status 81
NeXuS Transcatheter Mitral Chordal Repair Device – Product Description 81
Neochord Inc – Ongoing Clinical Trials Overview 82
NeoChord DS1000 – European First in Human Study of the NeoChord Transcatheter Mitral Repair System to Assess Safety and Performance in Patients With Symptomatic Mitral Regurgitation 83
NeoChord DS1000 – Randomized Trial of the NeoChord DS1000 System Versus Open Surgical Repair 83
NeoChord DS1000 – The AcChord Study: A Multicenter Post-market Observational Registry of the NeoChord Artificial Chordae Delivery System 83
NeXuS Transcatheter Mitral Chordal Repair Device – A US Early Feasibility Study of NeoChord Transcatheter NeXuS System in Patients with Mitral Valve Regurgitation 84
NewMed Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 85
Mi-thos Pro TMVR Valve – Product Status 85
Mi-thos Pro TMVR Valve – Product Description 85
Mi-thos Transcatheter Mitral Valve Replacement System – Product Status 86
Mi-thos Transcatheter Mitral Valve Replacement System – Product Description 86
NewMed Medical Co Ltd – Ongoing Clinical Trials Overview 87
Mi-thos Transcatheter Mitral Valve Replacement System – Evaluation of the Efficacy and Safety of the Mi-thos Transcatheter Mitral Valve Replacement System in Patients with Severe Mitral Valve Disease at High Surgical Risk 88
Mi-thos Transcatheter Mitral Valve Replacement System – Evaluation of the Efficacy and Safety of the Transcatheter Mitral Valve Repair System in Patients with Moderate and above Degenerative Mitral Regurgitation at High Surgical Risk 88
Mi-thos Transcatheter Mitral Valve Replacement System – Evaluation of the Efficacy and Safety of the Transcatheter Mitral Valve Repair System in the Treatment of Patients with Moderate and above Functional Mitral Regurgitation 88
Mi-thos Transcatheter Mitral Valve Replacement System – Pilot Study to Evaluate Transcatheter Mitral Valve Repair System in Patients with Moderate-to-severe Mitral Regurgitation at High Surgical Risk 89
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 90
Transapical Mitral Valve Regurgitation Device – Product Status 90
Transapical Mitral Valve Regurgitation Device – Product Description 90
Nyra Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 91
CARLEN Device – Product Status 91
CARLEN Device – Product Description 91
Open Stent Solution Pipeline Products & Ongoing Clinical Trials Overview 92
Open-Stent TMVR Device – Product Status 92
Open-Stent TMVR Device – Product Description 92
Peijia Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 93
Coaptation Augmentation Device – Product Status 93
Coaptation Augmentation Device – Product Description 93
GeminiOne TEER System – Product Status 94
GeminiOne TEER System – Product Description 94
Peijia TMVr – Product Status 94
Peijia TMVr – Product Description 95
Peijia TMVR SpyderOne – Product Status 95
Peijia TMVR SpyderOne – Product Description 95
Peijia Medical Ltd – Ongoing Clinical Trials Overview 96
GeminiOne TEER System – A Prospective, Multicentric Clinical Trial Protocol to Evaluate the Safety and Efficacy of the GeminiOne Transcatheter Valve Edge-to-Edge Repair System for the Treatment of Moderate-severe or Severe Degenerative Mitral Regurgitation with Single-arm Objective Performance Criteria 97
Pipeline Medical Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 98
Transcatheter Mitral Chordal Repair Device – Product Status 98
Transcatheter Mitral Chordal Repair Device – Product Description 98
Shanghai Hanyu Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 99
Second Generation ValveClamp – Product Status 99
Second Generation ValveClamp – Product Description 99
ValveClamp – Product Status 100
ValveClamp – Product Description 100
ValveClasp – Product Status 100
ValveClasp – Product Description 101
Shanghai Hanyu Medical Technology Co Ltd – Ongoing Clinical Trials Overview 102
ValveClamp – Multi-center Trial of Transcatheter Edge-to-edge Mitral Valve Repair with ValveClamp System in High Risk Patients with Degerative Mitral Regurgitation 103
ValveClasp – A Multicenter, Prospective, Single-arm Objective Performance Criteria Trial to Evaluate the Safety and Efficacy of the Transcatheter Mitral Valve Repair System: ValveClasp® in the Treatment of Moderate to Severe Mitral Regurgitation 104
Shanghai Huihe Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 105
M-Lock Mitral Valve Ring Repair System – Product Status 105
M-Lock Mitral Valve Ring Repair System – Product Description 105
M-Touch Mitral Valve Leaflet Repair System – Product Status 106
M-Touch Mitral Valve Leaflet Repair System – Product Description 106
Sutra Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 107
Sutra TMV Coaptation Augmentation System – Product Status 107
Sutra TMV Coaptation Augmentation System – Product Description 107
VALFIX Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 108
VALFIX System – Product Status 108
VALFIX System – Product Description 108
Valtech Cardio Ltd Pipeline Products & Ongoing Clinical Trials Overview 109
V-Chordal System – Transfemoral – Product Status 109
V-Chordal System – Transfemoral – Product Description 109
Vesalius Cardiovascular Inc Pipeline Products & Ongoing Clinical Trials Overview 110
Orion TMVR Device – Product Status 110
Orion TMVR Device – Product Description 110
Vvital Biomed Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Mitral Valve Posterior Leaflet Repair System – Product Status 111
Mitral Valve Posterior Leaflet Repair System – Product Description 111
Glossary 115
![]()