Tissue Engineered – Skin Substitutes Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Tissue engineering for wound care management is the use of mechanical and chemical processing of materials to manufacture products that may substitute or replace all or some components that make up normal skin (e.g., epidermis and/or dermis, cells, and matrix).
The tissue engineered – skin substitutes pipeline market research report provides comprehensive information about the tissue engineered – skin substitutes pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress. Moreover, the report provides information about various pipeline products and their estimated approval dates.
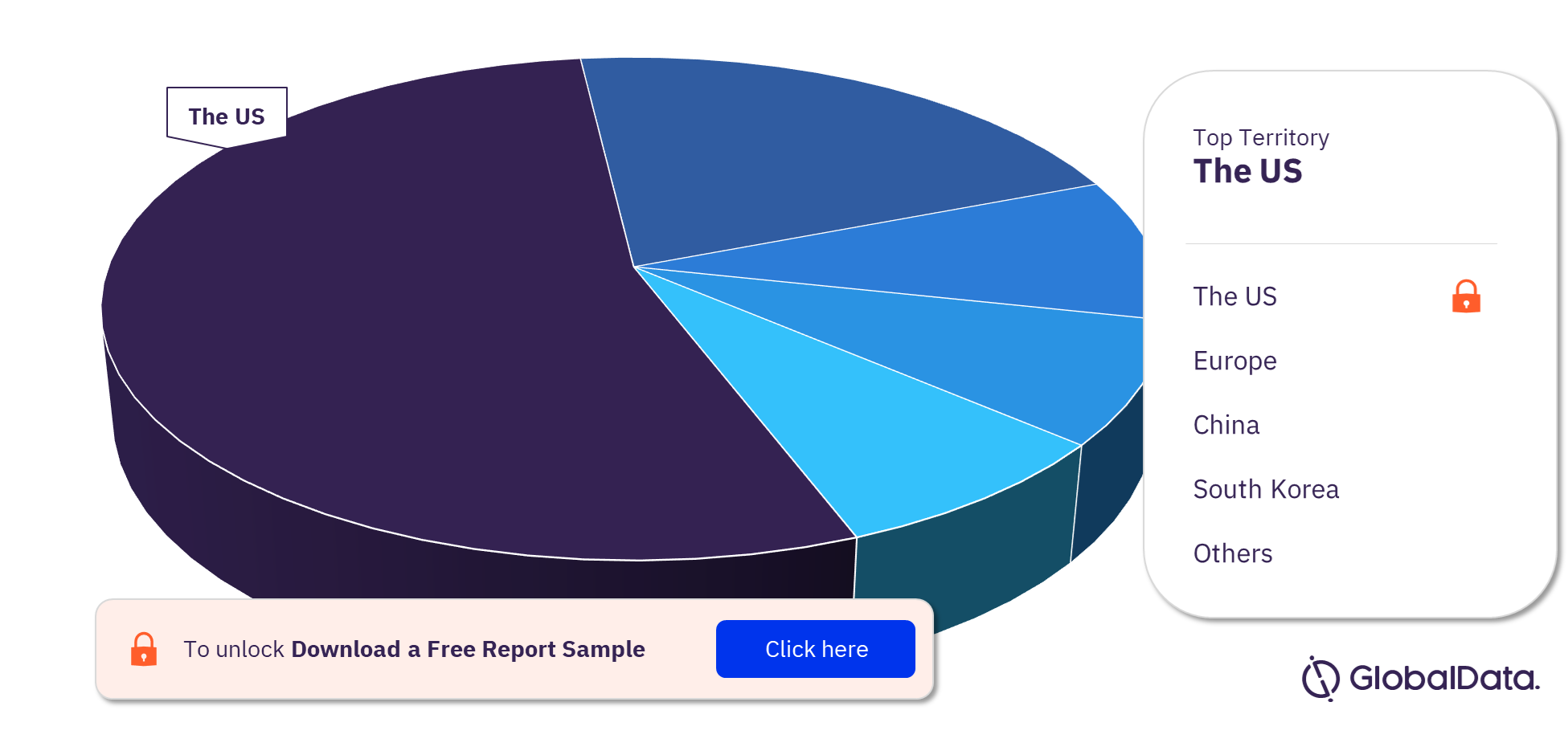
Tissue Engineered - Skin Substitutes Pipeline Products Market Segmentation by Territories
Some of the key territories with products in the pipeline are the US, Europe, China, South Korea, Taiwan, Japan, Kuwait, Malaysia, Mexico, and Mongolia. The US is the leading territory in the tissue engineered – skin substitutes pipeline products market followed by Europe and China.
Tissue Engineered – Skin Substitutes Pipeline Products Market Analysis by Territories
For more territory insights into the tissue engineered – skin substitutes pipeline products market, download a free report sample
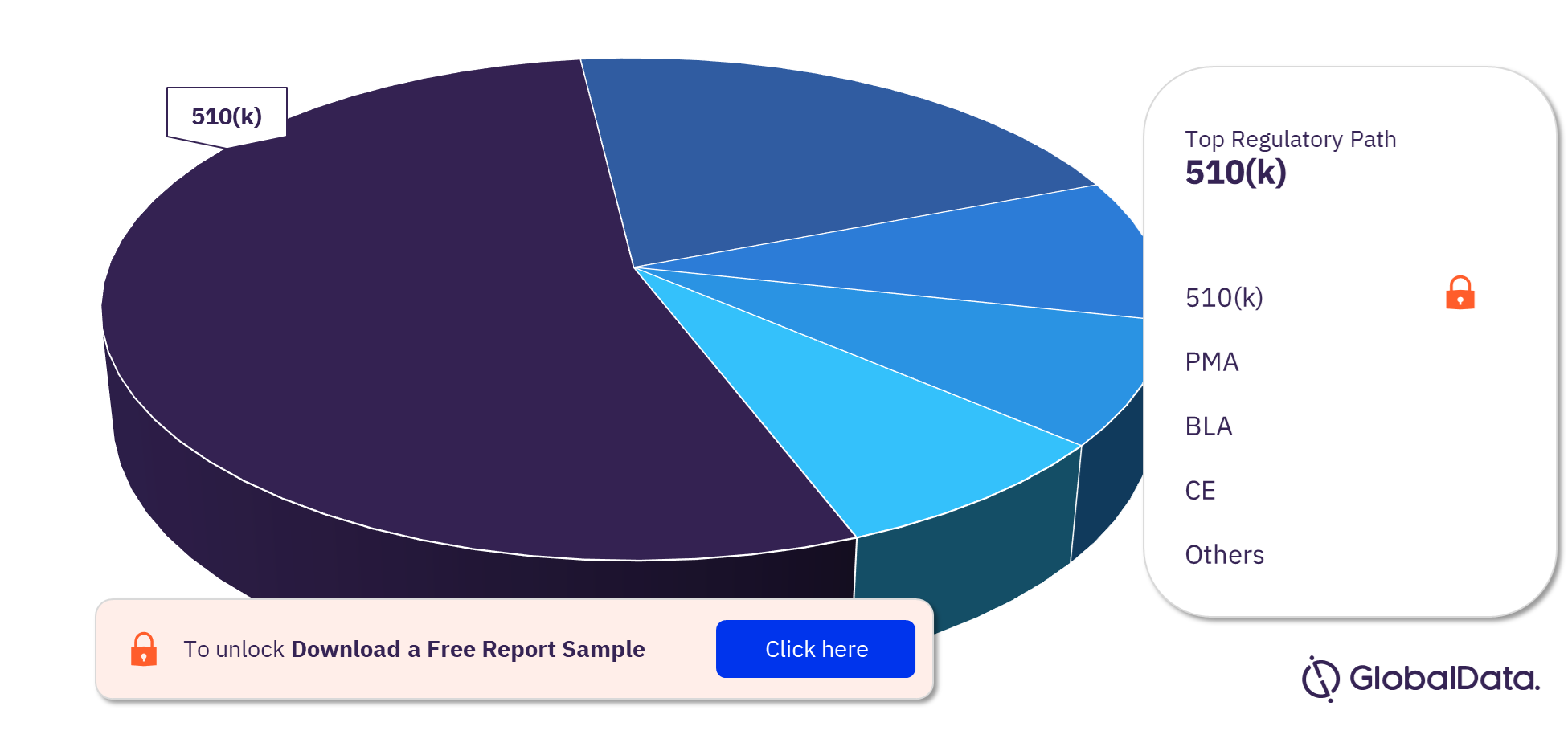
Tissue Engineered - Skin Substitutes Pipeline Products Market Segmentation by Regulatory Paths
The tissue engineered – skin substitutes pipeline report provides detailed insights into the pipeline products by a regulatory path including 510(k), PMA, BLA, CE, NMPA, BOPA, MDITAC, TGA, Shonin, and MDL, among others. 510(k) is the leading pathway.
Tissue Engineered – Skin Substitutes Pipeline Products Market Analysis by Regulatory Paths
For more regulatory path insights into the tissue engineered – skin substitutes pipeline products market, download a free report sample
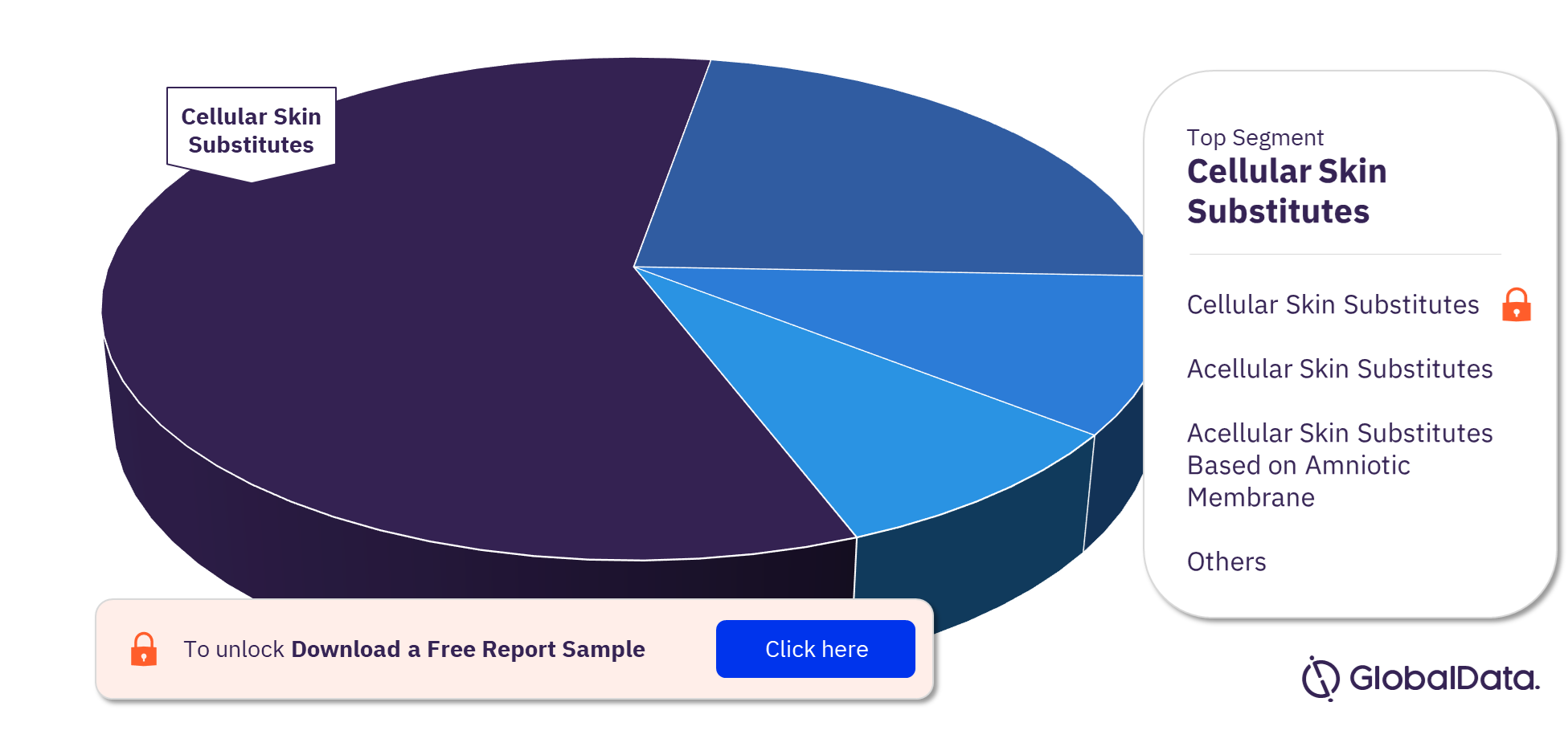
Tissue Engineered - Skin Substitutes Pipeline Products Market Segments
The key segments in the tissue engineered – skin substitutes pipeline products market are cellular skin substitutes, acellular skin substitutes, other acellular skin substitutes, acellular skin substitutes based on amniotic membrane, and cellular skin substitutes based on amniotic membrane. Cellular skin substitutes was the leading segment.
Cellular Skin Substitutes: They contain viable cells seeded onto the matrix material. They serve as a barrier to the environment and reduce inflammation and scarring by supporting wound closure without excessive fibrosis. This segment consists of cellular skin substitutes based on amniotic membranes and other cellular skin substitutes.
Acellular Skin Substitutes: They are made of materials similar to the host extracellular matrix and function as scaffolds. Acellular biomaterials can stimulate the micro-environment to heal wounds without the regulatory and scientific challenges of cellular treatment.
Tissue Engineered – Skin Substitutes Pipeline Products Market Analysis by Segments
For more segment insights into the tissue engineered – skin substitutes pipeline products market, download a free report sample
Tissue Engineered - Skin Substitutes Pipeline Products Market: Competitive Landscape
Some of the leading companies in the tissue engineered – skin substitutes pipeline products market are Acro Biomedical Co Ltd, Adept Medical Ltd, AGAM Biological Products, Amnio Technology LLC, Boston Children’s Hospital, China Regenerative Medicine International Ltd, Cutiss AG, Elanix Biotechnologies AG, Indian Institute of Technology Delhi, and Kerecis ehf.
Tissue Engineered - Skin Substitutes Pipeline Products Market Report Overview
| Key Territories | The US, Europe, China, South Korea, Taiwan, Japan, Kuwait, Malaysia, Mexico, and Mongolia |
| Key Regulatory Paths | 510(k), PMA, BLA, CE, NMPA, BOPA, MDITAC, TGA, Shonin, and MDL |
| Key Segments | Cellular Skin Substitutes, Acellular Skin Substitutes, Other Acellular Skin Substitutes, Acellular Skin Substitutes Based on Amniotic Membrane, and Cellular Skin Substitutes Based on Amniotic Membrane |
| Leading Companies | Acro Biomedical Co Ltd, Adept Medical Ltd, AGAM Biological Products, Amnio Technology LLC, Boston Children’s Hospital, China Regenerative Medicine International Ltd, Cutiss AG, Elanix Biotechnologies AG, Indian Institute of Technology Delhi, and Kerecis ehf |
Scope
- Extensive coverage of the tissue engineered – skin substitutes under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities
- The report reviews the major players involved in the development of tissue engineered – skin substitutes and lists all their pipeline projects
- The coverage of pipeline products is based on various stages of development ranging from early development to the approved/issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of tissue engineered – skin substitutes under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Adept Medical Ltd
AGAM Biological Products
Agenta Biotechnologies Inc (Inactive)
Amnio Technology LLC
Boston Children's Hospital
China Regenerative Medicine International Ltd
Cutiss AG
Elanix Biotechnologies AG
Indian Institute of Technology Delhi
Kerecis ehf
Lattice Biologics Ltd
LifeCell Corp
Merakris Therapeutics LLC
MiMedx Group Inc
Novadip Biosciences SA
Organogenesis Holdings Inc
Osiris Therapeutics Inc
PolarityTE Inc
PolyNovo Biomaterials Pty Ltd
Protein Genomics Inc
Purdue University
Regenicin Inc
Rensselaer Polytechnic Institute
ROKIT Healthcare Inc
Santai Biotech
Smith & Nephew Plc
SMSbiotech Inc
Sree Chitra Tirunal Institute for Medical Sciences & Technology
Stratatech Corp
Tel Aviv University
Tissue Regenix Ltd
University of Edinburgh
Vericel Corp
Worcester Polytechnic Institute
XenoTherapeutics Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the tissue engineered - skin substitutes pipeline products market?
Some of the key territories in the tissue engineered – skin substitutes pipeline products market are the US, Europe, China, South Korea, Taiwan, Japan, Kuwait, Malaysia, Mexico, and Mongolia.
-
What are the key regulatory paths in the tissue engineered - skin substitutes pipeline products market?
The key regulatory paths in the tissue engineered – skin substitutes pipeline products market are 510(k), PMA, BLA, CE, NMPA, BOPA, MDITAC, TGA, Shonin, and MDL, among others.
-
What are the key segments in the tissue engineered - skin substitutes pipeline products market?
The key segments in the tissue engineered – skin substitutes pipeline products market are cellular skin substitutes, acellular skin substitutes, other acellular skin substitutes, acellular skin substitutes based on amniotic membrane, and cellular skin substitutes based on amniotic membrane.
-
Which are the leading companies in the tissue engineered - skin substitutes pipeline products market?
Some of the leading companies in the tissue engineered – skin substitutes pipeline products market are Acro Biomedical Co Ltd, Adept Medical Ltd, AGAM Biological Products, Amnio Technology LLC, Boston Children’s Hospital, China Regenerative Medicine International Ltd, Cutiss AG, Elanix Biotechnologies AG, Indian Institute of Technology Delhi, and Kerecis ehf.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Wound Care Management reports