Thrombectomy Systems (Catheters) Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Thrombectomy Systems (Catheters) Pipeline Market Report Overview
Thrombectomy system involves the application of negative pressure for the removal of thrombi which is sometimes accompanied with thrombolytic drugs. The procedure is automated with the help of a console (or control unit) to which the catheter is attached. The Thrombectomy Systems (Catheters) pipeline market research report provides comprehensive information about the Thrombectomy Systems (Catheters) pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
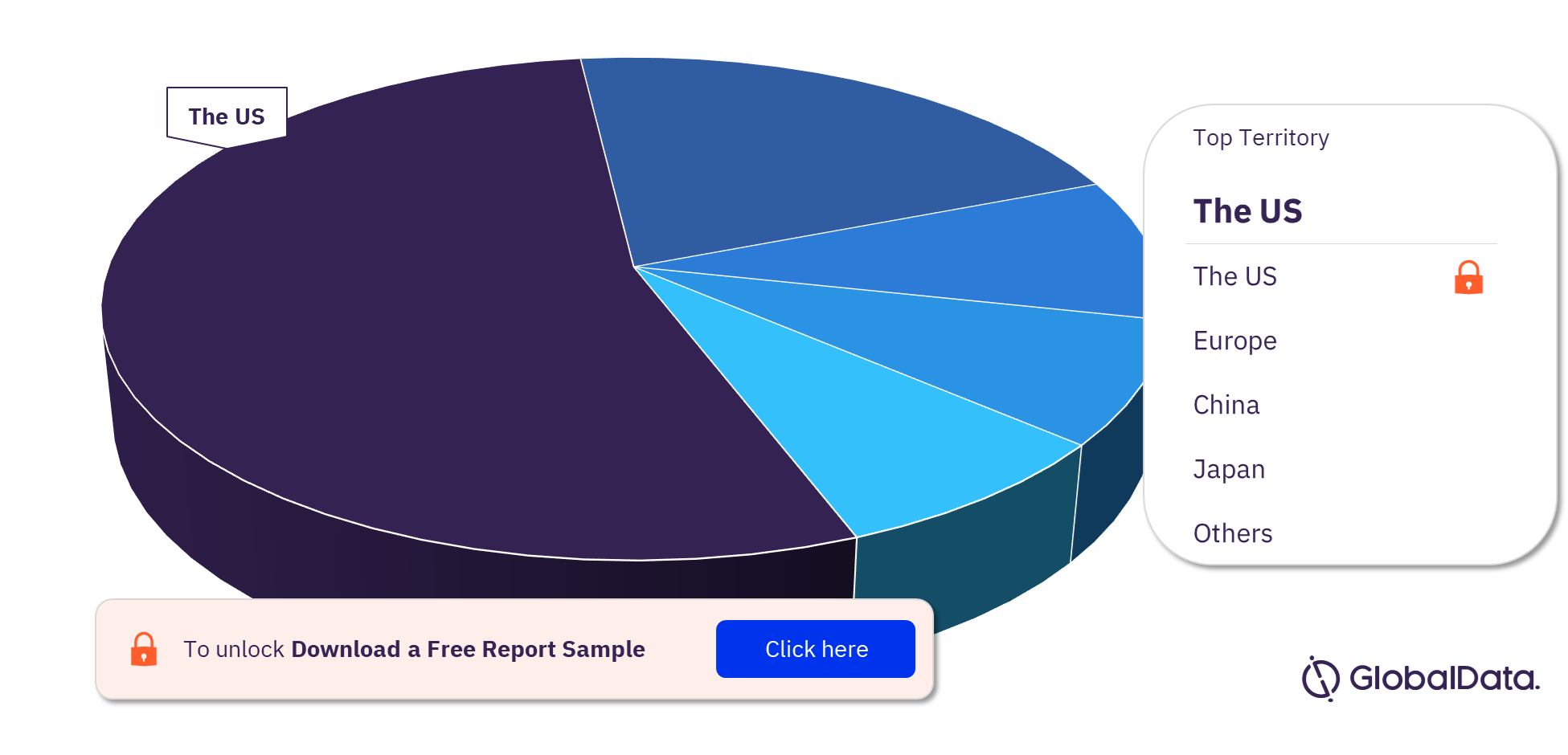
Thrombectomy Systems (Catheters) Pipeline Market Segmentation by Territories
Some of the key territories in the Thrombectomy Systems (Catheters)pipeline market are the US, China, Japan, Mexico, Brazil, Europe, and France. In 2023, the US has the highest number of pipeline products.
Thrombectomy Systems (Catheters) Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the Thrombectomy Systems (Catheters) pipeline market, download a free report sample
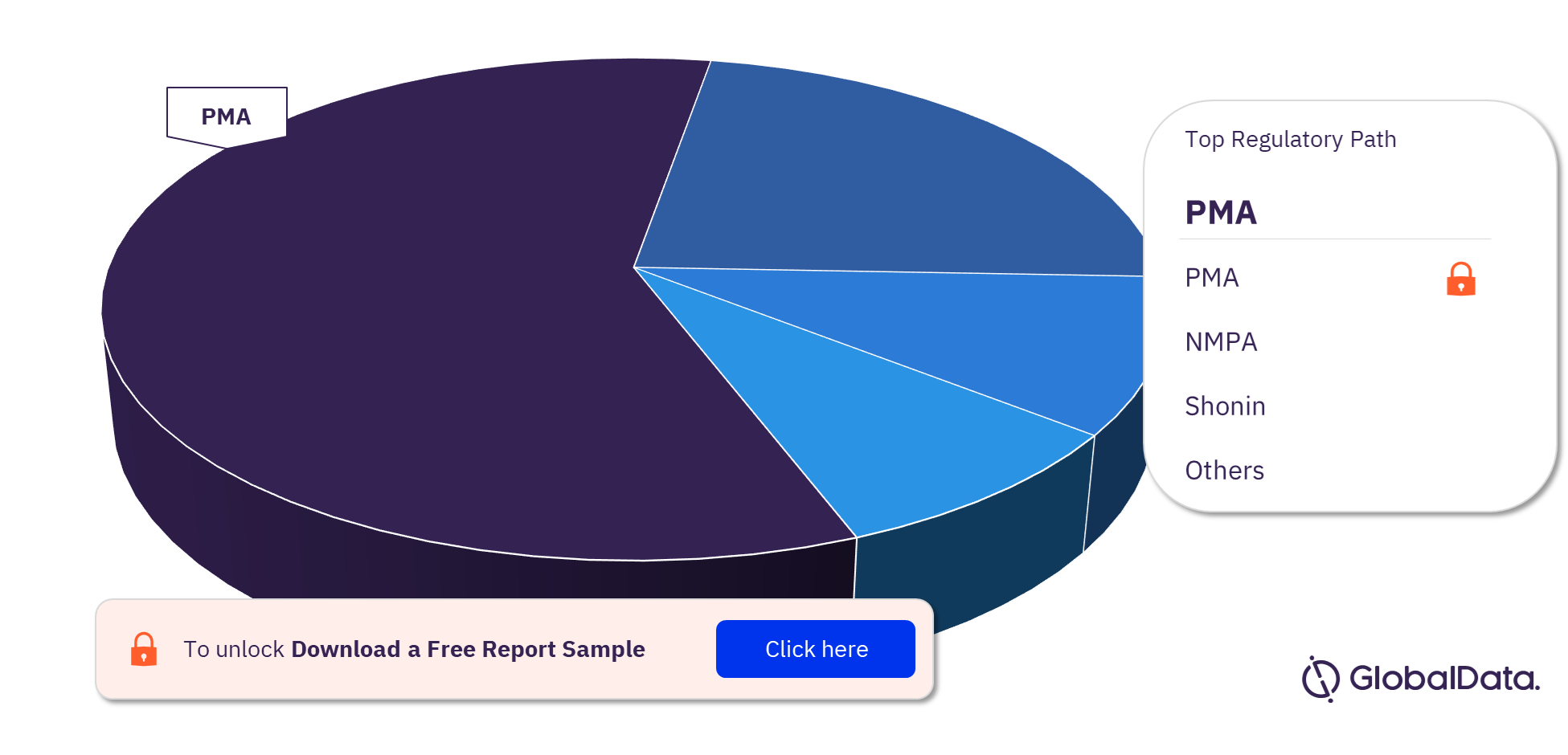
Thrombectomy Systems (Catheters) Pipeline Market Segmentation by Regulatory Paths
The key regulatory paths in the Thrombectomy Systems (Catheters) pipeline market are 510(k), NMPA, Ninsho, ANVISA, CE, PMA, and Shonin. In 2023, 510(k) was the most followed pathway for pipeline products.
Thrombectomy Systems (Catheters)Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory paths into the Thrombectomy Systems (Catheters) pipeline market, download a free report sample
Thrombectomy Systems (Catheters) Pipeline Market- Competitive Landscape
Some of the key companies in the Thrombectomy Systems (Catheters) pipeline market are AngioDynamics Inc, Boston Scientific Corp, Caeli Vascular LLC, Capture Vascular Inc, Inari Medical Inc, Innova Vascular Inc, Jiangsu Zhenyi Medical Technology Co Ltd, Liquet Medical Inc, Merit Medical Systems Inc, and MicroPort Scientific Corp.
Boston Scientific Corp: Headquartered in Marlborough, Massachusetts, the US, Boston Scientific Corp (Boston Scientific) is a medical technology company that is involved in the development, manufacturing and commercialization of devices for a range of interventional medical specialties. The company offers products in the areas of electrophysiology, gastroenterology, gastrointestinal surgery, female pelvic medicine, gynecology, interventional cardiology, interventional radiology, neurological surgery, orthopedic surgery, pain medicine, pulmonology, urology and vascular surgery.
Capture Vascular Inc: Headquartered in Mesa, Arizona, the US, Capture Vascular Inc (Capture Vascular) is a medical device development company that develops and commercializes mechanical thrombectomy systems. The company offers MegaVac, a mechanical thrombectomy system that removes clots in arteries and provides vessel occlusion, embolic prevention and anchoring capabilities. Its mechanical thrombectomy system is also designed to remove thrombus and emboli throughout the peripheral and coronary vasculature by occluding, disrupting and removing embolic and thrombotic materials.
Inari Medical Inc: Headquartered in Irvine, California, the US, Inari Medical Inc (Inari), formerly Inceptus Newco1 Inc, is a medical device company focused on developing products to treat venous diseases. The Company’s product offering consists of two minimally invasive, catheter-based mechanical thrombectomy devices. It offers solutions that enable the safe removal of clot volumes from big vessels.
Thrombectomy Systems (Catheters) Pipeline Market Report Overview
| Key Territories | The US, China, Japan, Mexico, Brazil, Europe, and France |
| Key Regulatory Paths | 510(k), NMPA, Ninsho, ANVISA, CE, PMA, and Shonin |
| Leading Companies | AngioDynamics Inc, Boston Scientific Corp, Caeli Vascular LLC, Capture Vascular Inc, Inari Medical Inc, Innova Vascular Inc, Jiangsu Zhenyi Medical Technology Co Ltd, Liquet Medical Inc, Merit Medical Systems Inc, and MicroPort Scientific Corp |
Segments Covered in the Report
Thrombectomy Systems (Catheters) Pipeline Market Territories Outlook
- The US
- China
- Japan
- Mexico
- Brazil
- Europe
- France
Thrombectomy Systems (Catheters) Pipeline Market Regulatory Paths Outlook
- 510(k)
- NMPA
- Ninsho
- ANVISA
- CE
- PMA
- Shonin
Scope
- Extensive coverage of the Thrombectomy Systems (Catheters) under development.
- Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates.
- Reviews of major players involved in the pipeline product development.
- Provides key clinical trial data related to ongoing clinical trials such as trial phase, trial status, trial start and end dates, and the number of trials of the major Thrombectomy Systems (Catheters) pipeline products.
- Review of Recent Developments in the segment / industry.
Reasons to Buy
The Thrombectomy Systems (Catheters) report enables you to:
- Access significant competitor information, analysis, and insights to improve your R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of Thrombectomy Systems (Catheters) under development.
- Formulate market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
Boston Scientific Corp
Caeli Vascular LLC
Capture Vascular Inc
Inari Medical Inc
Innova Vascular Inc
Jiangsu Zhenyi Medical Technology Co Ltd
Liquet Medical Inc
Merit Medical Systems Inc
MicroPort Scientific Corp
Morningside (Nantong) Medical Co Ltd
NexGen Medical Systems Inc
OrbusNeich
Penumbra Inc
Shanghai Changde Medical Technology Co Ltd
Shanghai MicroPort Endovascular MedTech Co Ltd
Suzhou Tianhong Shengjie Medical Co Ltd
TransMed7 LLC
University of California Los Angeles
University of Toledo
University of Wolverhampton
Wallaby Medical Inc
WaveClear Inc
Zylox-Tonbridge Medical Technology Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the Thrombectomy Systems (Catheters) pipeline market?
Some of the key territories in the Thrombectomy Systems (Catheters) pipeline market are the US, China, Japan, Mexico, Brazil, Europe, and France.
-
What are the key regulatory paths in the Thrombectomy Systems (Catheters) pipeline market?
The key regulatory paths in the Thrombectomy Systems (Catheters) pipeline market are 510(k), NMPA, Ninsho, ANVISA, CE, PMA, and Shonin.
-
What is the use of Thrombectomy system?
Thrombectomy system involves the application of negative pressure for the removal of thrombi which is sometimes accompanied with thrombolytic drugs.
-
Which are the leading companies in the Thrombectomy Systems (Catheters) pipeline market?
Some of the leading companies in the Thrombectomy Systems (Catheters) pipeline market are AngioDynamics Inc, Boston Scientific Corp, Caeli Vascular LLC, Capture Vascular Inc, Inari Medical Inc, Innova Vascular Inc, Jiangsu Zhenyi Medical Technology Co Ltd, Liquet Medical Inc, Merit Medical Systems Inc, and MicroPort Scientific Corp.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Thrombectomy Systems (Catheters) reports