Thoracic Aortic Stent Grafts Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Thoracic Aortic Stent Grafts Pipeline Market Report Overview
Thoracic aortic stent grafts are used to treat thoracic aortic aneurysms by reinforcing a part of the aorta using a minimally invasive procedure. The Thoracic Aortic Stent Grafts pipeline market research report provides comprehensive information about the Thoracic Aortic Stent Grafts pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
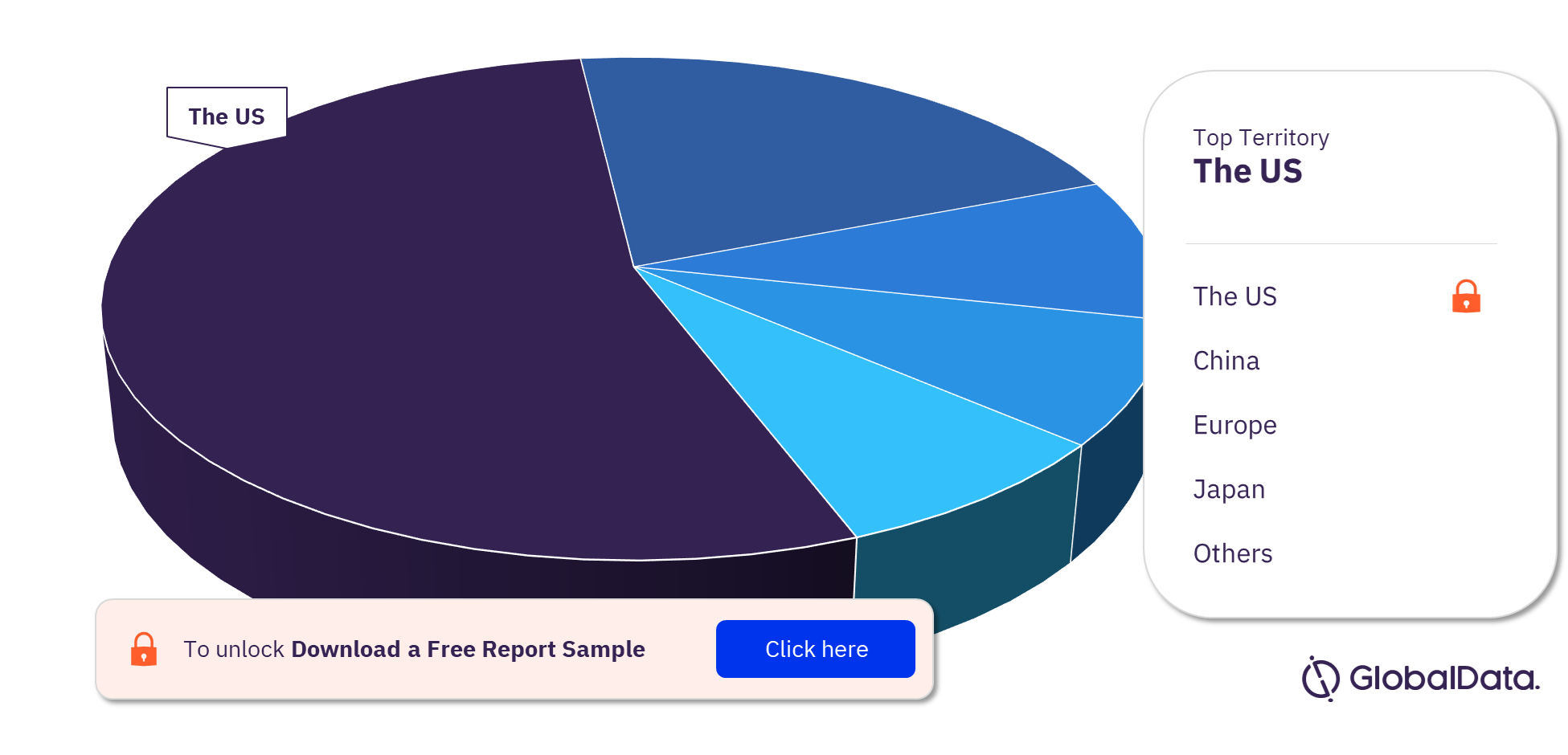
Thoracic Aortic Stent Grafts Pipeline Market Segmentation by Territories
Some of the key territories in the Thoracic Aortic Stent Grafts pipeline market are the US, China, Europe, Japan, and Canada. In 2023, the US has the highest number of pipeline products.
Thoracic Aortic Stent Grafts Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the Thoracic Aortic Stent Grafts pipeline market, download a free report sample
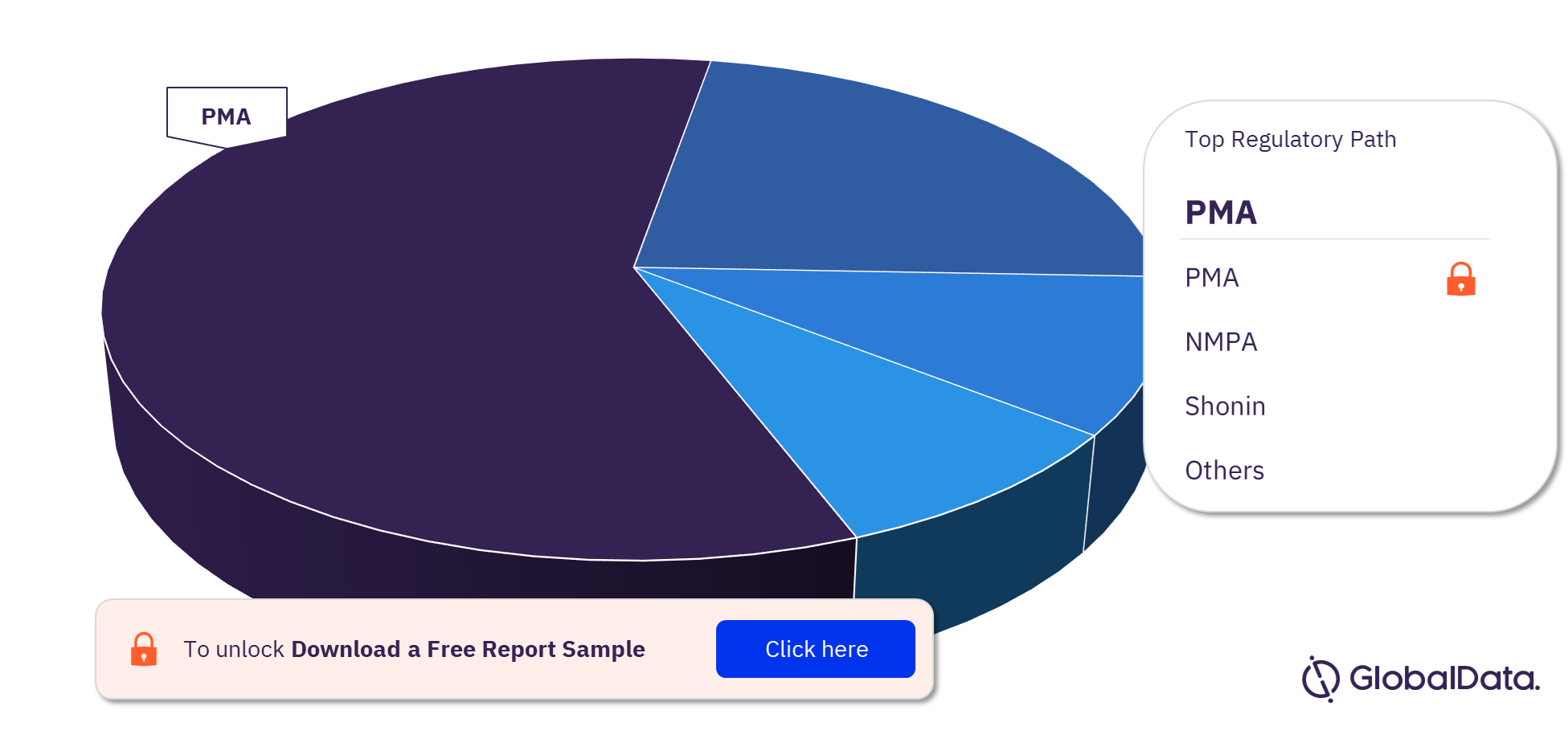
Thoracic Aortic Stent Grafts Pipeline Market Segmentation by Regulatory Paths
The key regulatory paths in the Thoracic Aortic Stent Grafts pipeline market are PMA, NMPA, Shonin, CE, and MDL. In 2023, PMA was the most followed regulatory path for pipeline products.
Thoracic Aortic Stent Grafts Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory path insights into the Thoracic Aortic Stent Grafts pipeline market, download a free report sample
Thoracic Aortic Stent Grafts Pipeline Market – Competitive Landscape
Some of the key companies in the Thoracic Aortic Stent Grafts pipeline market are APT Medical Inc, Artivion Inc, Beijing Tianzhu Ruichang Medical Technology Co Ltd, EndoSpan Ltd, Evasc Medical Systems Corp, Hangzhou Endonom Medtech Co Ltd, JOTEC GmbH, Lombard Medical Technologies Inc., Lundquist, and Medtronic Plc.
APT Medical Inc: Headquartered in Nanshan District, Shenzhen, China, APT Medical Inc is a supplier and manufacturer of EP and vascular intervention medical devices.
Artivion Inc: Headquartered in Atlanta, Georgia, the US, Artivion Inc (Artivion), formerly known as CryoLife Inc, is a medical device company that manufactures and distributes biosurgical devices and implantable human tissues for cardiac and vascular surgical procedures for the treatment of aortic disease. The company’s portfolio enlists preserved human cardiac allografts, vascular allografts, surgical adhesives and sealants, heart valves, bovine pericardium, and mitral chordal repair products, photofix products and Jotec products. The company products serve physicians, cardiac surgeons, hospitals, and medical institutions.
Beijing Tianzhu Ruichang Medical Technology Co Ltd: Beijing Tianzhu Ruichang Medical Technology Co Ltd is a medical device company which focusses on the development and production of innovative aortic stent graft systems.
Thoracic Aortic Stent Grafts Pipeline Market Report Overview
| Key Territories | The US, China, Europe, Japan, and Canada |
| Key Regulatory Paths | PMA, NMPA, Shonin, CE, and MDL |
| Leading Companies | APT Medical Inc, Artivion Inc, Beijing Tianzhu Ruichang Medical Technology Co Ltd, EndoSpan Ltd, Evasc Medical Systems Corp, Hangzhou Endonom Medtech Co Ltd, JOTEC GmbH, Lombard Medical Technologies Inc., Lundquist, and Medtronic Plc. |
Segments Covered in the Report
Thoracic Aortic Stent Grafts Pipeline Market Territories Outlook
- The US
- China
- Europe
- Japan
- Canada
Thoracic Aortic Stent Grafts Pipeline Market Regulatory Paths Outlook
- PMA
- NMPA
- Shonin
- CE
- MDL
Scope
- Extensive coverage of the Thoracic Aortic Stent Grafts under development.
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities.
- The report reviews the major players involved in the development of Thoracic Aortic Stent Grafts and list all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from early development to approved / issued stage.
- The report provides key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to –
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of Thoracic Aortic Stent Grafts under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory and estimated launch date.
Artivion Inc
Beijing Tianzhu Ruichang Medical Technology Co Ltd
EndoSpan Ltd
Evasc Medical Systems Corp
Hangzhou Endonom Medtech Co Ltd
JOTEC GmbH
Lombard Medical Technologies Inc.
Lundquist
Medtronic Plc
Sree Chitra Tirunal Institute for Medical Sciences & Technology
St. Jude Medical LLC
Terumo Aortic
TriVascular Inc
Zylox-Tonbridge Medical Technology Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the Thoracic Aortic Stent Grafts pipeline market?
Some of the key territories in the Thoracic Aortic Stent Grafts pipeline market are the US, China, Europe, Japan, and Canada.
-
What are the key regulatory paths in the Thoracic Aortic Stent Grafts pipeline market?
The key regulatory paths in the Thoracic Aortic Stent Grafts pipeline market are PMA, NMPA, Shonin, CE, and MDL.
-
What are Thoracic aortic stent grafts used for?
Thoracic aortic stent grafts are used to treat thoracic aortic aneurysms by reinforcing a part of the aorta using a minimally invasive procedure.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Thoracic Aortic Stent Grafts reports