Mechanical Circulatory Support Devices – Pipeline Products by Stage of Development 18
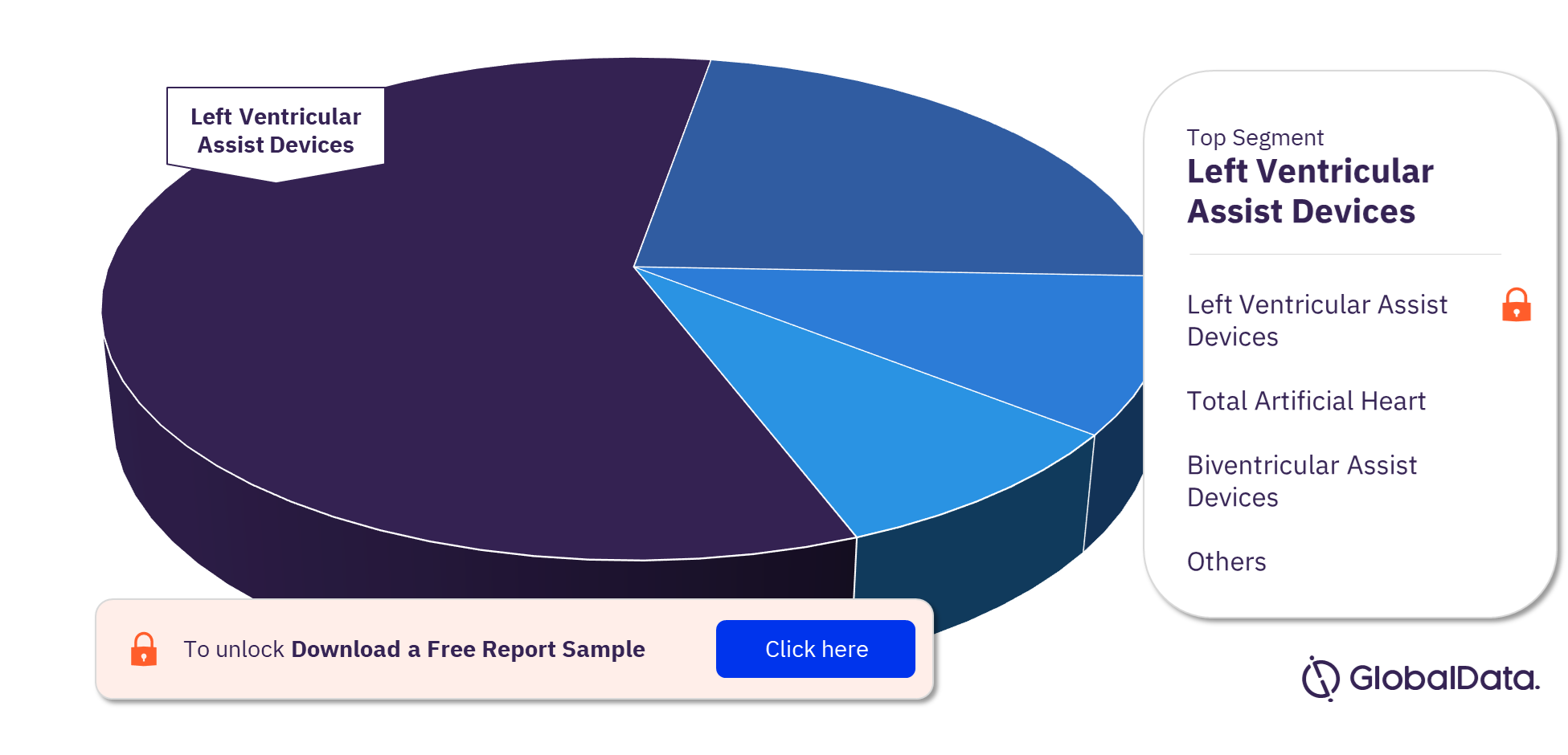
Mechanical Circulatory Support Devices – Pipeline Products by Segment 19
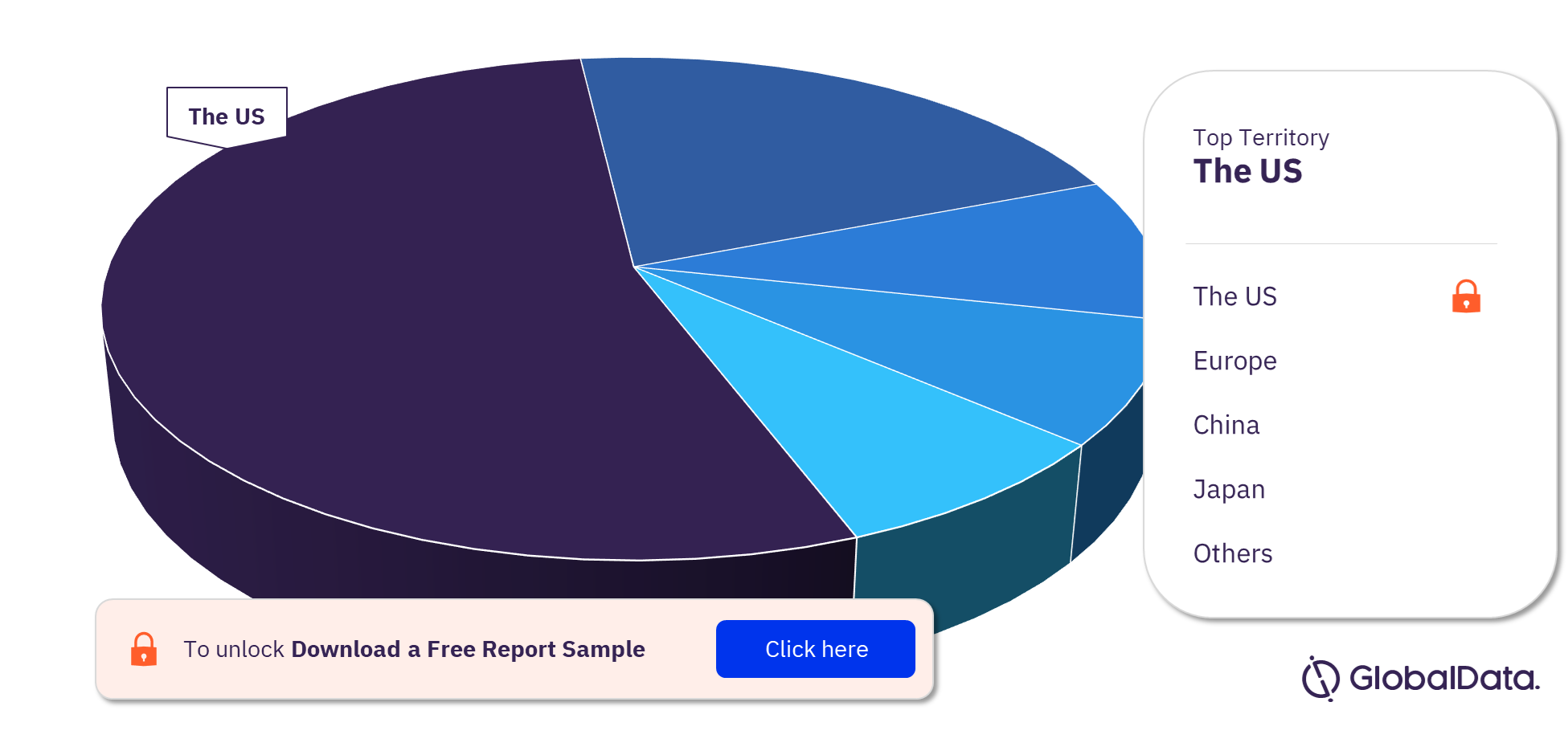
Mechanical Circulatory Support Devices – Pipeline Products by Territory 21
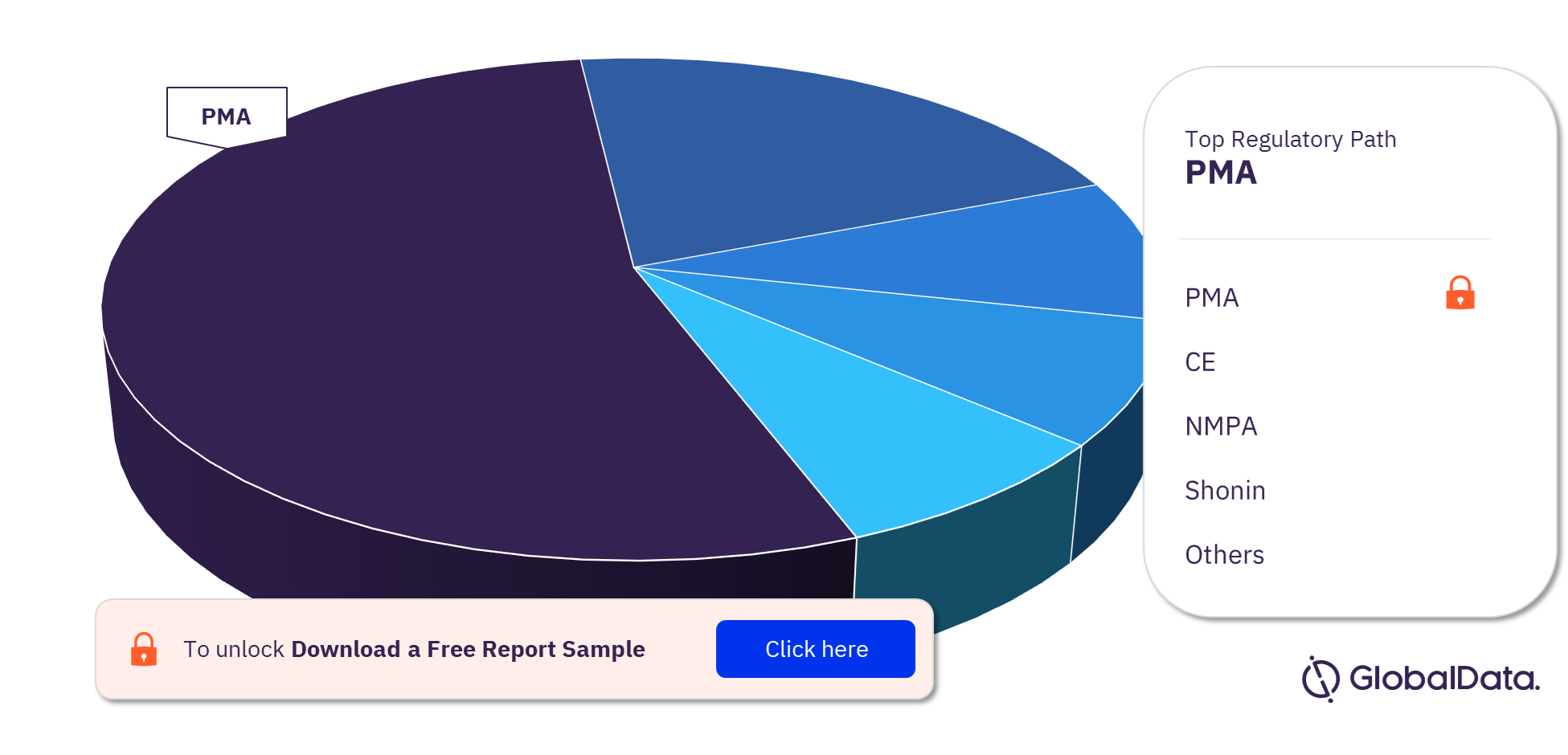
Mechanical Circulatory Support Devices – Pipeline Products by Regulatory Path 22
Mechanical Circulatory Support Devices – Pipeline Products by Estimated Approval Date 23
Mechanical Circulatory Support Devices – Ongoing Clinical Trials 24
Mechanical Circulatory Support Devices Companies – Pipeline Products by Stage of Development 25
Mechanical Circulatory Support Devices – Pipeline Products by Stage of Development 29
Abbott Laboratories Pipeline Products & Ongoing Clinical Trials Overview 33
Fully Implantable Left Ventricular Assist System – Product Status 33
Fully Implantable Left Ventricular Assist System – Product Description 33
Abiomed Inc Pipeline Products & Ongoing Clinical Trials Overview 34
AbioCor II – Product Status 34
AbioCor II – Product Description 35
Impella BTR – Product Status 35
Impella BTR – Product Description 35
Impella ECP – Product Status 36
Impella ECP – Product Description 36
Impella RP IJ – Product Status 36
Impella RP IJ – Product Description 37
Next Generation Impella CP – STEMI – Product Status 37
Next Generation Impella CP – STEMI – Product Description 37
Abiomed Inc – Ongoing Clinical Trials Overview 38
Impella ECP – Use of the Impella ECP in Patients Undergoing an Elective High-risk Percutaneous Coronary Intervention: An Early Feasibility Study 39
Impella ECP – Use of the Impella ECP in Patients Undergoing an Elective or Urgent High-Risk Percutaneous Coronary Intervention 39
Next Generation Impella CP – STEMI – Primary Unloading and Delayed Reperfusion in St-elevation Myocardial Infarction: The STEMI-DTU Trial 40
Impella BTR – Use of the Impella BTR in Patients with Heart Failure: An Early Feasibility Study 41
AdjuCor GmbH Pipeline Products & Ongoing Clinical Trials Overview 42
reBEAT – Product Status 42
reBEAT – Product Description 42
APK Advanced Medical Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 43
Apical Access System – Product Status 43
Apical Access System – Product Description 43
Arrow International Cr, A.S. Pipeline Products & Ongoing Clinical Trials Overview 44
CorAide Left Ventricular Assist System – Product Status 44
CorAide Left Ventricular Assist System – Product Description 44
BioLife4D Corp Pipeline Products & Ongoing Clinical Trials Overview 45
Mini-Heart – Product Status 45
Mini-Heart – Product Description 45
BioVentrix Inc Pipeline Products & Ongoing Clinical Trials Overview 46
PliCath HF – Product Status 46
PliCath HF – Product Description 46
BiVacor Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 47
BiVACOR Device – Product Status 47
BiVACOR Device – Product Description 47
Braile Biomedica Ltda Pipeline Products & Ongoing Clinical Trials Overview 48
Blood Pump – Product Status 48
Blood Pump – Product Description 48
Calon Cardio-Technology Ltd Pipeline Products & Ongoing Clinical Trials Overview 49
Calon MiniVAD – Product Status 49
Calon MiniVAD – Product Description 49
CathVAD – Product Status 50
CathVAD – Product Description 50
MicroVAD – Product Status 50
MicroVAD – Product Description 51
Cardiac Success Ltd Pipeline Products & Ongoing Clinical Trials Overview 52
V-Sling Device – Product Status 52
V-Sling Device – Product Description 52
CardiacAssist Inc Pipeline Products & Ongoing Clinical Trials Overview 53
Pediatric pVAD – Product Status 53
Pediatric pVAD – Product Description 53
CardiacBooster BV Pipeline Products & Ongoing Clinical Trials Overview 54
Percutaneous Ventricular Assist Device – Product Status 54
Percutaneous Ventricular Assist Device – Product Description 54
CARDIAnove Inc Pipeline Products & Ongoing Clinical Trials Overview 55
CARDIAnove Pump – Product Status 55
CARDIAnove Pump – Product Description 55
Cardiovascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 56
MCS System – Product Status 56
MCS System – Product Description 56
Carmat SAS Pipeline Products & Ongoing Clinical Trials Overview 57
Aeson – Product Status 57
Aeson – Product Description 58
Carmat SAS – Ongoing Clinical Trials Overview 59
Aeson – Multicentric Prospective Cohort Study in Patients with Irreversible Biventricular Heart Failure to Assess the Efficacy and Safety of Carmat TAH, Its Clinical Utility and Cost, as a Bridge to Transplantation 60
Carnegie Mellon University Pipeline Products & Ongoing Clinical Trials Overview 61
Non-Blood Contacting Muscle Powered VAD – Product Status 61
Non-Blood Contacting Muscle Powered VAD – Product Description 61
tVAD – Heart Failure – Product Status 62
tVAD – Heart Failure – Product Description 62
Cleveland Clinic Hospital Pipeline Products & Ongoing Clinical Trials Overview 63
PediPump – Product Status 63
PediPump – Product Description 63
Cleveland Clinic Lerner College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 64
Continuous-Flow Total Artificial Heart – Product Status 64
Continuous-Flow Total Artificial Heart – Product Description 64
Pediatric Continuous-Flow Total Artificial Heart – Product Status 65
Pediatric Continuous-Flow Total Artificial Heart – Product Description 65
Cleveland Heart Inc Pipeline Products & Ongoing Clinical Trials Overview 66
SmartHeart LVAD – Product Status 66
SmartHeart LVAD – Product Description 66
SmartHeart TAH – Product Status 67
SmartHeart TAH – Product Description 67
Colorado State University Pipeline Products & Ongoing Clinical Trials Overview 68
Flexible Multilayered Pump – Product Status 68
Flexible Multilayered Pump – Product Description 68
CorAssist Cardiovascular Ltd Pipeline Products & Ongoing Clinical Trials Overview 69
CORolla – Product Status 69
CORolla – Product Description 69
ImCardia – Product Status 70
ImCardia – Product Description 70
CorAssist Cardiovascular Ltd – Ongoing Clinical Trials Overview 71
CORolla – CORolla TAA for Heart Failure with Preserved Ejection Fraction (HFpEF) and Diastolic Dysfunction (DD) Safety and Feasibility 72
CorInnova, Inc. Pipeline Products & Ongoing Clinical Trials Overview 73
Cardiacstar – Bridge To Decision – Product Status 73
Cardiacstar – Bridge To Decision – Product Description 73
Cardiacstar – Bridge To Recovery – Product Status 74
Cardiacstar – Bridge To Recovery – Product Description 74
Cardiacstar – Bridge To Transplant – Product Status 74
Cardiacstar – Bridge To Transplant – Product Description 75
Cardiacstar – Destination Therapy – Product Status 75
Cardiacstar – Destination Therapy – Product Description 75
Cardiacstar – Heart Rehabilitation – Product Status 76
Cardiacstar – Heart Rehabilitation – Product Description 76
Epicheart – Soft Robotic Heart Device – Product Status 76
Epicheart – Soft Robotic Heart Device – Product Description 77
CoRISMA MCS Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 78
CoRISMA – Product Status 78
CoRISMA – Product Description 78
Corvion Inc Pipeline Products & Ongoing Clinical Trials Overview 79
Nautilus Pump – Product Status 79
Nautilus Pump – Product Description 79
CorWave SA Pipeline Products & Ongoing Clinical Trials Overview 80
CorWave LVAD – Product Status 80
CorWave LVAD – Product Description 80
CorWave Nemo – Product Status 81
CorWave Nemo – Product Description 81
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 82
Intravascular Blood Pump – Product Status 82
Intravascular Blood Pump – Product Description 82
Duke University Pipeline Products & Ongoing Clinical Trials Overview 83
Para-Aortic Blood Pump – Product Status 83
Para-Aortic Blood Pump – Product Description 83
Ension, Inc. Pipeline Products & Ongoing Clinical Trials Overview 84
CardioFlowPQ – Product Status 84
CardioFlowPQ – Product Description 84
Magnetic Levitation Motor – Product Status 85
Magnetic Levitation Motor – Product Description 85
Evaheart Inc Pipeline Products & Ongoing Clinical Trials Overview 86
EVAHEART 2 Left Ventricular Assist System – Product Status 86
EVAHEART 2 Left Ventricular Assist System – Product Description 86
Evaheart Inc – Ongoing Clinical Trials Overview 87
EVAHEART 2 Left Ventricular Assist System – Prospective Multi-Center Randomized Study for Evaluating the EVAHEART2 Left Ventricular Assist System: the COMPETENCE Trial 88
FineHeart Pipeline Products & Ongoing Clinical Trials Overview 89
ICOMS FLOWMAKER – Product Status 89
ICOMS FLOWMAKER – Product Description 89
Foster-Miller Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 90
Magscrew Total Artificial Heart – Product Status 90
Magscrew Total Artificial Heart – Product Description 90
Fundacion para la Investigacion Biomedica del Hospital Gregorio Maranon Pipeline Products & Ongoing Clinical Trials Overview 91
Ventricular Assist Device – Product Status 91
Ventricular Assist Device – Product Description 91
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 92
OptiFlo – Product Status 92
OptiFlo – Product Description 92
Griffith University Pipeline Products & Ongoing Clinical Trials Overview 93
Next Generation Artificial Heart – Product Status 93
Next Generation Artificial Heart – Product Description 93
Grupo Vitalmex SA de CV Pipeline Products & Ongoing Clinical Trials Overview 94
Vitacor – UVAD – Product Status 94
Vitacor – UVAD – Product Description 94
Hangzhou Shengshi Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 95
Mechanical Circulation Auxiliary Device – Product Status 95
Mechanical Circulation Auxiliary Device – Product Description 95
HeartWare International Inc Pipeline Products & Ongoing Clinical Trials Overview 96
BiVAD – Product Status 96
BiVAD – Product Description 96
HeartWare IV-VAD – Product Status 97
HeartWare IV-VAD – Product Description 97
HVAD System – Destination Therapy – Product Status 97
HVAD System – Destination Therapy – Product Description 98
Levacor Rotary VAD – Product Status 98
Levacor Rotary VAD – Product Description 98
Longhorn – Product Status 99
Longhorn – Product Description 99
MiFlow VAD – Product Status 99
MiFlow VAD – Product Description 100
Miniaturized Ventricular Assist Device (MVAD) Pump – Product Status 100
Miniaturized Ventricular Assist Device (MVAD) Pump – Product Description 100
VCAN Trans-Mitral – Product Status 101
VCAN Trans-Mitral – Product Description 101
HeartWare International Inc – Ongoing Clinical Trials Overview 102
Miniaturized Ventricular Assist Device (MVAD) Pump – Multi Center, Prospective, Non-randomized, Single-arm Trial Evaluating the Clinical Safety and Performance of the HeartWare MVAD System for the Treatment of Advanced Heart Failure 103
I M Sechenov Moscow State Medical University Pipeline Products & Ongoing Clinical Trials Overview 104
Cardiac Assist Device – Children – Product Status 104
Cardiac Assist Device – Children – Product Description 104
Indian Institute of Technology, Kanpur Pipeline Products & Ongoing Clinical Trials Overview 105
Left Ventricular Assist Device – Product Status 105
Left Ventricular Assist Device – Product Description 105
Indiana University Pipeline Products & Ongoing Clinical Trials Overview 106
Bi-Conical Heart Pump – Product Status 106
Bi-Conical Heart Pump – Product Description 106
Innova Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 107
Mechanical Circulatory Support System – Product Status 107
Mechanical Circulatory Support System – Product Description 107
Inspired Therapeutics LLC Pipeline Products & Ongoing Clinical Trials Overview 108
Neomate System – Product Status 108
Neomate System – Product Description 108
Jarvik Heart Inc Pipeline Products & Ongoing Clinical Trials Overview 109
Jarvik 2000 Heart – Bridge To Transplant – Product Status 109
Jarvik 2000 Heart – Bridge To Transplant – Product Description 109
Jarvik 2000 Heart – Destination Therapy – Product Status 110
Jarvik 2000 Heart – Destination Therapy – Product Description 110
Jarvik 2015 – Product Status 110
Jarvik 2015 – Product Description 111
Jarvik Heart Inc – Ongoing Clinical Trials Overview 112
Jarvik 2000 Heart – Destination Therapy – Evaluation of the Jarvik 2000 Left Ventricular Assist System with Post-auricular Connector–destination Therapy Study 113
Jarvik 2015 – Pumps for Kids, Infants, and Neonates 114
LaunchPoint Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 115
Pediaflow Pediatric VAD – Product Status 115
Pediaflow Pediatric VAD – Product Description 115
Streamliner VAD – Product Status 116
Streamliner VAD – Product Description 116
Lausanne University Hospital Pipeline Products & Ongoing Clinical Trials Overview 117
RollingHeart – Product Status 117
RollingHeart – Product Description 117
Leviticus Cardio Ltd Pipeline Products & Ongoing Clinical Trials Overview 118
FiVAD System – Product Status 118
FiVAD System – Product Description 118
Hybrid FILVAS – Product Status 119
Hybrid FILVAS – Product Description 119
Levram Medical Systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 120
Levram – Ventricular Assist Device – Product Status 120
Levram – Ventricular Assist Device – Product Description 120
Magenta Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 121
Magenta pLVAD – Product Status 121
Magenta pLVAD – Product Description 121
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 122
Cardiac Device – Diastolic Heart Failure – Product Status 122
Cardiac Device – Diastolic Heart Failure – Product Description 122
Implantable Left Ventricular Assist Device – Product Status 123
Implantable Left Ventricular Assist Device – Product Description 123
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 124
Fully Implantable Left Ventricular Assist Device – Product Status 124
Fully Implantable Left Ventricular Assist Device – Product Description 124
Michigan Critical Care Consultants Inc Pipeline Products & Ongoing Clinical Trials Overview 125
BioVAD – Product Status 125
BioVAD – Product Description 125
Pediatric Mpulse – Product Status 126
Pediatric Mpulse – Product Description 126
MyoCardioCare, Inc. Pipeline Products & Ongoing Clinical Trials Overview 127
MCC3000 Heart Pump – Product Status 127
MCC3000 Heart Pump – Product Description 127
MCC3000 Heart Pump – Pediatrics – Product Status 128
MCC3000 Heart Pump – Pediatrics – Product Description 128
Myotech, LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 129
Myotech Circulatory Support System – Product Status 129
Myotech Circulatory Support System – Product Description 129
NeoCardial Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 130
eHeart Extra Ventricular Assist Device – Product Status 130
eHeart Extra Ventricular Assist Device – Product Description 130
Nipro Corp Pipeline Products & Ongoing Clinical Trials Overview 131
BR16010 – Product Status 131
BR16010 – Product Description 131
Implantable Left Ventricular Assist Device – Product Status 132
Implantable Left Ventricular Assist Device – Product Description 132
Novapump GmbH Pipeline Products & Ongoing Clinical Trials Overview 133
PERKAT Biventricular Heart Pump – Product Status 133
PERKAT Biventricular Heart Pump – Product Description 133
PERKAT RV – Product Status 134
PERKAT RV – Product Description 134
NuPulseCV, Inc. Pipeline Products & Ongoing Clinical Trials Overview 135
NuPulseCV iVAS – Product Status 135
NuPulseCV iVAS – Product Description 135
PediPulse – Product Status 136
PediPulse – Product Description 136
NuPulseCV, Inc. – Ongoing Clinical Trials Overview 137
NuPulseCV iVAS – A Clinical Study to Evaluate Ambulatory Counterpulsation for the Treatment of Advanced Heart Failure: A Feasibility Study 138
OregonHeart Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 139
Next Generation Total Artificial Heart – Product Status 139
Next Generation Total Artificial Heart – Product Description 139
Pennsylvania State University Pipeline Products & Ongoing Clinical Trials Overview 140
Pediatric Heart-Assist Device – Product Status 140
Pediatric Heart-Assist Device – Product Description 140
Percassist Inc Pipeline Products & Ongoing Clinical Trials Overview 141
PercAssist Ventricular Assist Device – Product Status 141
PercAssist Ventricular Assist Device – Product Description 141
Perfusion Solution Inc Pipeline Products & Ongoing Clinical Trials Overview 142
Cavopulmonary Assist System – Product Status 142
Cavopulmonary Assist System – Product Description 142
Right Ventricular Assist Device – Long Term Care – Product Status 143
Right Ventricular Assist Device – Long Term Care – Product Description 143
Transapical LVAD – Product Status 143
Transapical LVAD – Product Description 144
PlugMed Heart SAS Pipeline Products & Ongoing Clinical Trials Overview 145
Implantable Left Ventricular Assist Device – Product Status 145
Implantable Left Ventricular Assist Device – Product Description 145
Procyrion Inc Pipeline Products & Ongoing Clinical Trials Overview 146
Aortix Device – Product Status 146
Aortix Device – Product Description 146
Procyrion Inc – Ongoing Clinical Trials Overview 147
Aortix Device – An Evaluation of the Safety and Performance of the Aortix System for Intra-aortic Mechanical Circulatory Support in Patients with Cardiorenal Syndrome 148
Aortix Device – Aortix Therapy for Perioperative Reduction of Renal Injury 148
PulseCath BV Pipeline Products & Ongoing Clinical Trials Overview 149
iVAC 2L – Product Status 149
iVAC 2L – Product Description 150
Purdue University Pipeline Products & Ongoing Clinical Trials Overview 151
Viscous Impeller Pump – Infants – Product Status 151
Viscous Impeller Pump – Infants – Product Description 151
Puzzle Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 152
ModulHeart – Product Status 152
ModulHeart – Product Description 152
ReliantHeart, Inc. Pipeline Products & Ongoing Clinical Trials Overview 153
aVAD Liberty System – Product Status 153
aVAD Liberty System – Product Description 153
Pulse Less Total Artificial Heart – Product Status 154
Pulse Less Total Artificial Heart – Product Description 154
VAD Maintenance System – Product Status 154
VAD Maintenance System – Product Description 155
RT Cardiac Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 156
Percutaneous Mechanical Circulatory Support System – Product Status 156
Percutaneous Mechanical Circulatory Support System – Product Description 156
Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 157
Temporary Percutaneous Ventricular Assist Device – Product Status 157
Temporary Percutaneous Ventricular Assist Device – Product Description 157
Saint Louis University Pipeline Products & Ongoing Clinical Trials Overview 158
Percutaneously Implantable Cardiovascular Support Device – Product Status 158
Percutaneously Implantable Cardiovascular Support Device – Product Description 158
TURBOCARDIA – Product Status 159
TURBOCARDIA – Product Description 159
Scandinavian Real Heart AB Pipeline Products & Ongoing Clinical Trials Overview 160
Total Artificial Heart – Product Status 160
Total Artificial Heart – Product Description 160
Total Artificial Heart – Clinical Version – Product Status 161
Total Artificial Heart – Clinical Version – Product Description 161
Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 162
Paracorporeal Left Ventricle Assist Device – Product Status 162
Paracorporeal Left Ventricle Assist Device – Product Description 162
St. Jude Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 163
UltraMag Adult Ventricular Assist System – Product Status 163
UltraMag Adult Ventricular Assist System – Product Description 163
UltraMag Pediatric Ventricular Assist System – Product Status 164
UltraMag Pediatric Ventricular Assist System – Product Description 164
Sunshine Heart Company, Pty. Ltd. Pipeline Products & Ongoing Clinical Trials Overview 165
C-Pulse – Wireless Version – Product Status 165
C-Pulse – Wireless Version – Product Description 165
Supira Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 166
Next Generation Percutaneous Ventricular Assist Device – Product Status 166
Next Generation Percutaneous Ventricular Assist Device – Product Description 166
Suzhou Xinqing Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 167
External Magnetic Levitation Artificial Heart – Product Status 167
External Magnetic Levitation Artificial Heart – Product Description 167
Syncardia Systems LLC Pipeline Products & Ongoing Clinical Trials Overview 168
10cc Ventricular Assist Device – Product Status 168
10cc Ventricular Assist Device – Product Description 168
30cc Ventricular Assist Device – Product Status 169
30cc Ventricular Assist Device – Product Description 169
60cc Ventricular Assist Device – Product Status 169
60cc Ventricular Assist Device – Product Description 170
SynCardia 50cc Temporary Total Artificial Heart – Destination Therapy – Product Status 170
SynCardia 50cc Temporary Total Artificial Heart – Destination Therapy – Product Description 170
SynCardia 70cc Temporary Total Artificial Heart – Destination Therapy – Product Status 171
SynCardia 70cc Temporary Total Artificial Heart – Destination Therapy – Product Description 171
Syncardia Systems LLC – Ongoing Clinical Trials Overview 172
SynCardia 70cc Temporary Total Artificial Heart – Destination Therapy – SynCardia 70cc Temporary Total Artificial Heart (TAH-t) for Destination Therapy (DT) 173
Syntach AB Pipeline Products & Ongoing Clinical Trials Overview 174
Syntach CS – Product Status 174
Syntach CS – Product Description 174
TEDA International Cardiovascular Hospital Pipeline Products & Ongoing Clinical Trials Overview 175
Heartcon – Product Status 175
Heartcon – Product Description 175
The Artificial Heart Laboratory of Foundation for Cardiac Surgery Development Pipeline Products & Ongoing Clinical Trials Overview 176
ReligaHeart EXT – Product Status 176
ReligaHeart EXT – Product Description 176
Thoratec LLC Pipeline Products & Ongoing Clinical Trials Overview 177
DuraHeart II – Product Status 177
DuraHeart II – Product Description 177
Fully Implantable Ventricular Assist System – Product Status 178
Fully Implantable Ventricular Assist System – Product Description 178
HeartMate X – Product Status 178
HeartMate X – Product Description 179
Tokyo Medical and Dental University Pipeline Products & Ongoing Clinical Trials Overview 180
TinyPump – Product Status 180
TinyPump – Product Description 180
U.S. Stem Cell Inc Pipeline Products & Ongoing Clinical Trials Overview 181
SVAD – Product Status 181
SVAD – Product Description 181
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 182
Ventricular Assist Device Monitor – Product Status 182
Ventricular Assist Device Monitor – Product Description 182
University of Louisville Pipeline Products & Ongoing Clinical Trials Overview 183
Paediatric Heart Pump – Product Status 183
Paediatric Heart Pump – Product Description 183
University of Tokyo Pipeline Products & Ongoing Clinical Trials Overview 184
Helical Flow Total Artificial Heart – Product Status 184
Helical Flow Total Artificial Heart – Product Description 184
University of Virginia Pipeline Products & Ongoing Clinical Trials Overview 185
VADStent – Product Status 185
VADStent – Product Description 185
Vadovations Inc Pipeline Products & Ongoing Clinical Trials Overview 186
REVOLUTION – Product Status 186
REVOLUTION – Product Description 186
VASCOR Inc Pipeline Products & Ongoing Clinical Trials Overview 187
Vascor LVAD – Product Status 187
Vascor LVAD – Product Description 187
Windmill Cardiovascular Systems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 188
Pediatric TORVAD – Product Status 188
Pediatric TORVAD – Product Description 188
TORVAD – Product Status 189
TORVAD – Product Description 189
W-Z BIOTECH, LLC Pipeline Products & Ongoing Clinical Trials Overview 190
Double Lumen Cannula-Based Left Ventricular Assist Device – Product Status 190
Double Lumen Cannula-Based Left Ventricular Assist Device – Product Description 190
SppVAD – Product Status 191
SppVAD – Product Description 191
Yale University Pipeline Products & Ongoing Clinical Trials Overview 192
BoLetz Pump – Product Status 192
BoLetz Pump – Product Description 192
CAP RVAD Pump – Product Status 193
CAP RVAD Pump – Product Description 193
Glossary 220
![]()