Left Ventricular Assist Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Left Ventricular Assist Devices Pipeline Market Report Overview
An implantable left ventricular assist device is a ventricular assist system intended for the treatment of end-stage heart failure. It assists the left ventricle by pumping oxygenated blood to the body. It can also be used to treat the right ventricle in some cases.
The left ventricular assist devices pipeline market research report provides comprehensive information about the left ventricular assist devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress. Moreover, the report provides information about various pipeline products and their estimated approval dates.
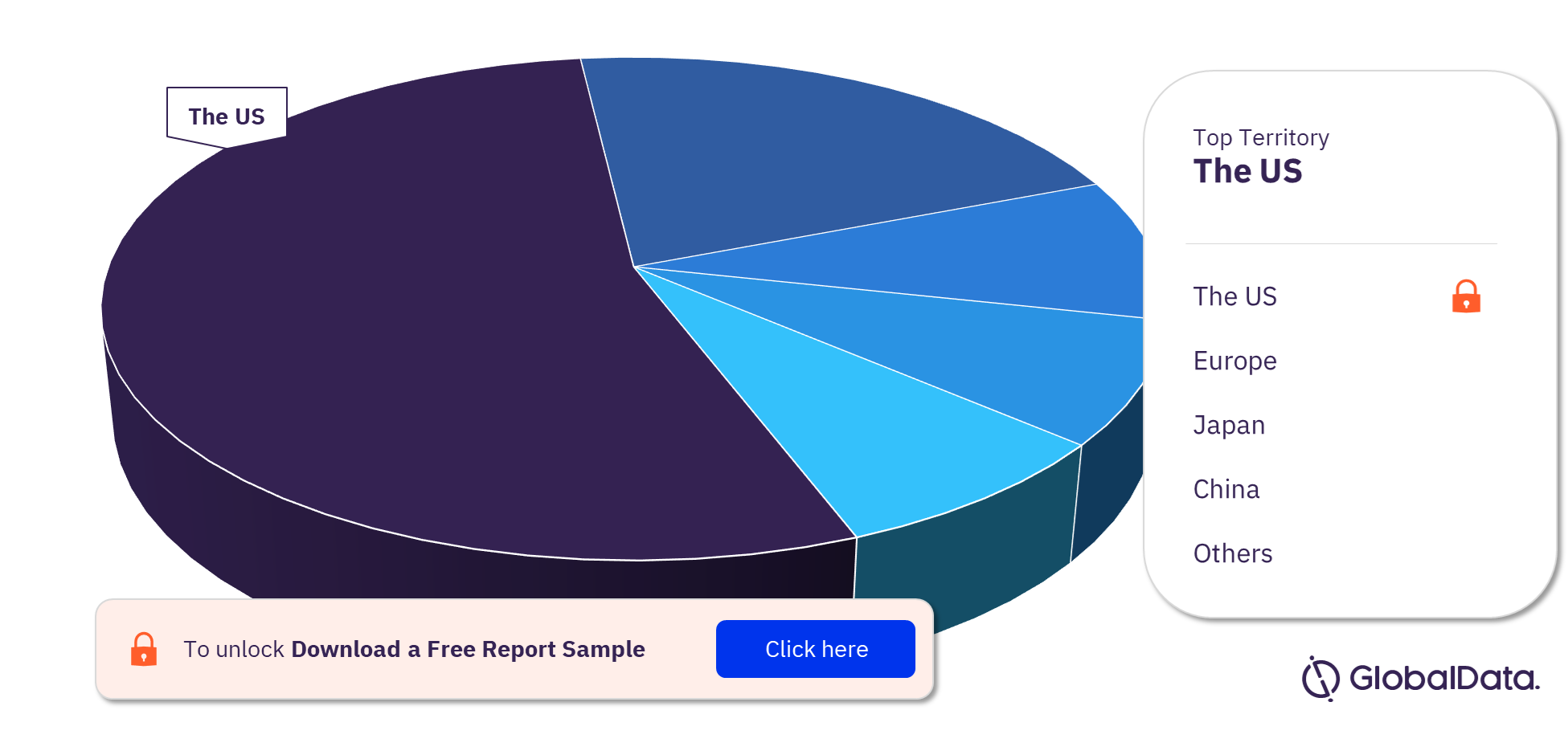
Left Ventricular Assist Devices Pipeline Products Market Segmentation by Territories
Some of the key territories with products in the pipeline are the US, Europe, Japan, China, India, Israel, Canada, Singapore, South Korea, and Taiwan among others. The US is the leading territory in the left ventricular assist devices pipeline products market as of February 2023.
Left Ventricular Assist Devices Pipeline Products Market Analysis by Territories, 2023 (%)
For more territory insights into the left ventricular assist devices pipeline products market, download a free report sample
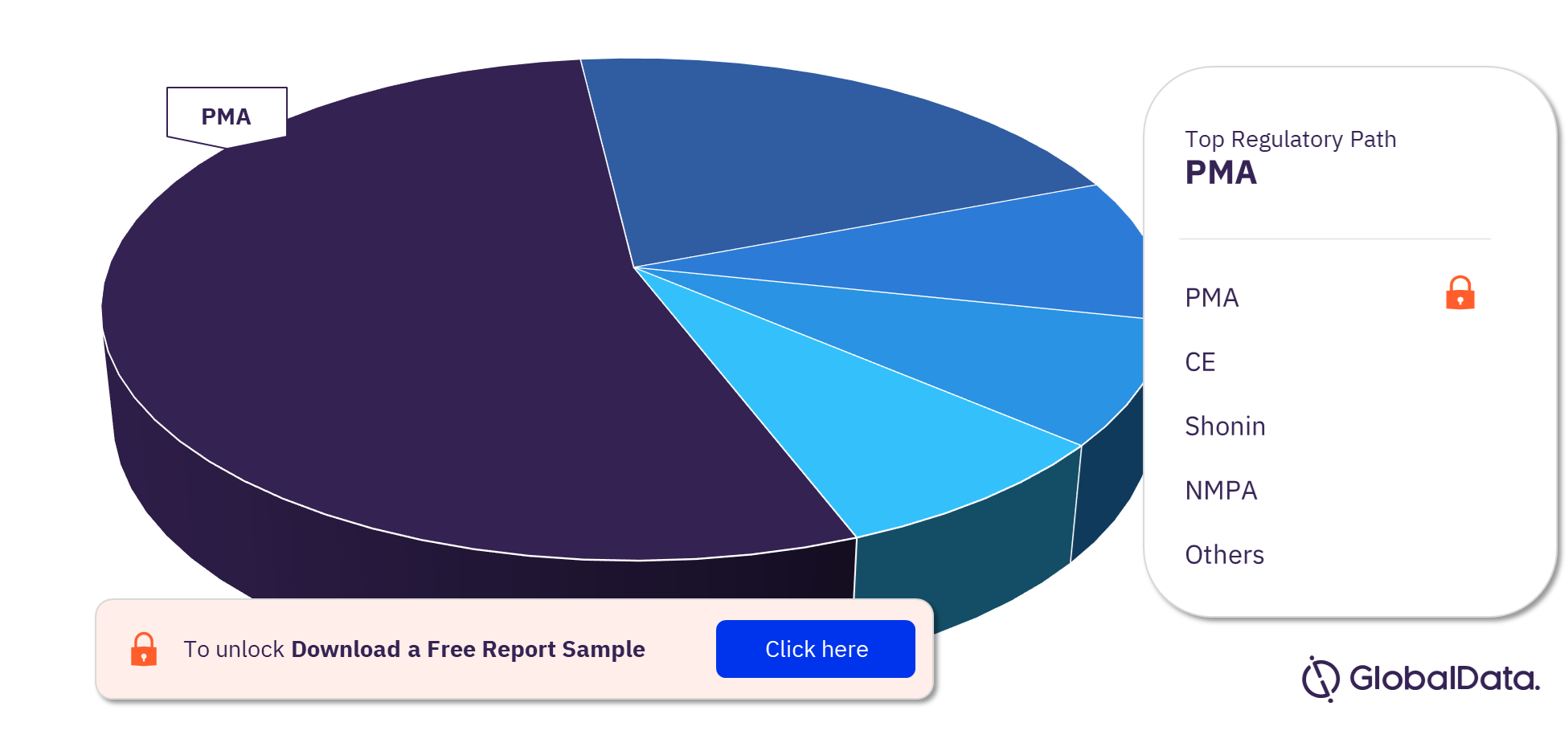
Left Ventricular Assist Devices Pipeline Products Market Segmentation by Regulatory Paths
The left ventricular assist devices pipeline report provides detailed insights into the pipeline products by a regulatory path including PMA, CE, Shonin, NMPA, ICAC, MDL, HDE Approvals, HSA, and 510(k) among others. Most of the products follow the PMA pathway to enter the market.
Left Ventricular Assist Devices Pipeline Products Market Analysis by Regulatory Paths, 2023 (%)
For more regulatory path insights into the left ventricular assist devices pipeline products market, download a free report sample
Left Ventricular Assist Devices Pipeline Products Market - Competitive Landscape
Some of the leading companies in the left ventricular assist devices pipeline products market are Abbott Laboratories, Abiomed Inc, APK Advanced Medical Technologies Inc., Arrow International Cr, A.S., BioVentrix Inc, Calon Cardio-Technology Ltd, Cardiac Success Ltd, CardiacBooster BV, CARDIAnove Inc, and Carnegie Mellon University.
Abbott Laboratories: Abbott Laboratories (Abbott) discovers, develops, manufactures, and sells a diversified range of healthcare products including branded generic pharmaceuticals, diagnostic systems & tests, and pediatric & adult nutritional products. The company also offers various medical devices including heart failure, electrophysiology, rhythm management, vascular & structural heart devices, and neuromodulation devices.
Abiomed Inc: Abiomed Inc (Abiomed) is a medical device company that develops, manufactures, and markets circulatory support devices. The devices replace or assist the pumping function in a failing heart. The company’s heart support and recovery product portfolio include Impella heart pumps (catheter-based devices including micro heart pumps, expandable catheter pumps, and axial flow pumps) and Breethe system (implantable cardiac and respiratory assist devices).
For more insights on the leading players in the left ventricular assist devices pipeline products market, download a free report sample
Left Ventricular Assist Devices Pipeline Products Market Report Overview
| Key Territories | The US, Europe, Japan, China, India, Israel, Canada, Singapore, South Korea, and Taiwan |
| Key Regulatory Paths | PMA, CE, Shonin, NMPA, ICAC, MDL, HDE Approvals, HSA, and 510(k) |
| Leading Companies | Abbott Laboratories, Abiomed Inc, APK Advanced Medical Technologies Inc., Arrow International Cr, A.S., BioVentrix Inc, Calon Cardio-Technology Ltd, Cardiac Success Ltd, CardiacBooster BV, CARDIAnove Inc, and Carnegie Mellon University |
Left Ventricular Assist Devices Pipeline Products Territorial Outlook (Number of Products, 2023)
- The US
- Europe
- Japan
- China
- India
- Israel
- Canada
- Singapore
- South Korea
- Taiwan
Left Ventricular Assist Devices Pipeline Products Regulatory Paths Outlook (Number of Products, 2023)
- PMA
- CE
- Shonin
- NMPA
- ICAC
- MDL
- HDE Approvals
- HAS
- 510(k)
Scope
- Extensive coverage of the left ventricular assist devices under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities
- The report reviews the major players involved in the development of left ventricular assist devices and lists all their pipeline projects
- The coverage of pipeline products is based on various stages of development ranging from early development to the approved/issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of left ventricular assist devices under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Abiomed Inc
APK Advanced Medical Technologies, Inc.
Arrow International Cr, A.S.
BioVentrix Inc
Calon Cardio-Technology Ltd
Cardiac Success Ltd
CardiacBooster BV
CARDIAnove Inc
Carnegie Mellon University
Cleveland Clinic Hospital
Cleveland Heart Inc
CorAssist Cardiovascular Ltd
Corvion Inc
CorWave SA
Duke University
Evaheart Inc
FineHeart
HeartWare International Inc
Indian Institute of Technology, Kanpur
Inspired Therapeutics LLC
Jarvik Heart Inc
LaunchPoint Technologies Inc
Leviticus Cardio Ltd
Levram Medical Systems Ltd
Magenta Medical Inc
Mayo Clinic
Medtronic Plc
Nipro Corp
OrbusNeich
Percassist Inc
Perfusion Solution Inc
PlugMed Heart SAS
PulseCath BV
ReliantHeart, Inc.
Shenzhen Core Medical Technology Co Ltd
Sree Chitra Tirunal Institute for Medical Sciences & Technology
Sunshine Heart Company, Pty. Ltd.
Syntach AB
Thoratec LLC
Tokyo Medical and Dental University
U.S. Stem Cell Inc
University of Virginia
VASCOR Inc
Windmill Cardiovascular Systems, Inc.
W-Z BIOTECH, LLC
Yale University
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the left ventricular assist devices pipeline products market?
Some of the key territories in the left ventricular assist devices pipeline products market are the US, Europe, Japan, China, India, Israel, Canada, Singapore, South Korea, and Taiwan.
-
Which is the leading territory in the left ventricular assist devices pipeline products market?
The US is the leading territory in the left ventricular assist devices pipeline products market.
-
What are the key regulatory paths in the left ventricular assist devices pipeline products market?
The key regulatory paths in the left ventricular assist devices pipeline products market are PMA, CE, Shonin, NMPA, ICAC, MDL, HDE Approvals, HSA, and 510(k) among others.
-
Which are the leading companies in the left ventricular assist devices pipeline products market?
Some of the leading companies in the left ventricular assist devices pipeline products market are Abbott Laboratories, Abiomed Inc, APK Advanced Medical Technologies Inc., Arrow International Cr, A.S., BioVentrix Inc, Calon Cardio-Technology Ltd, Cardiac Success Ltd, CardiacBooster BV, CARDIAnove Inc, and Carnegie Mellon University.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Left Ventricular Assist Devices reports