Intraocular Lens (IOL) Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Intraocular Lens (IOL) Pipeline Market Report Overview
Intraocular lens is an artificial lens made of plastic, silicone, acrylic or other material that is implanted inside the eye during cataract surgery. Foldable (phakic) IOL and Polymethyl Methacrylate (PMMA) IOL are tracked under this category.
The Intraocular Lens (IOL) pipeline market research report provides comprehensive information about the IOL pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · Polymethyl Methacrylate (PMMA) IOL
· Foldable IOL · Monofocal Foldable IOL · Multifocal Foldable IOL |
| Key Territories | · The US
· Europe · China · Japan · India · Canada · Australia · New Zealand · Singapore · Israel |
| Key Regulatory Paths | · PMA
· CE · NMPA · Shonin · ICAC · MDL · HDE Approvals · HAS · TGA · 510(k) |
| Leading Companies | · Adaptilens LLC
· Adoptics AG (Inactive) · AkkoLens International BV · Alcon Inc · Anew Optics, Inc. · Atia Vision Inc · Bausch & Lomb Inc · Care Group · CEYEBER Corp · Clarvista Medical Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
IOL Pipeline Market by Segments
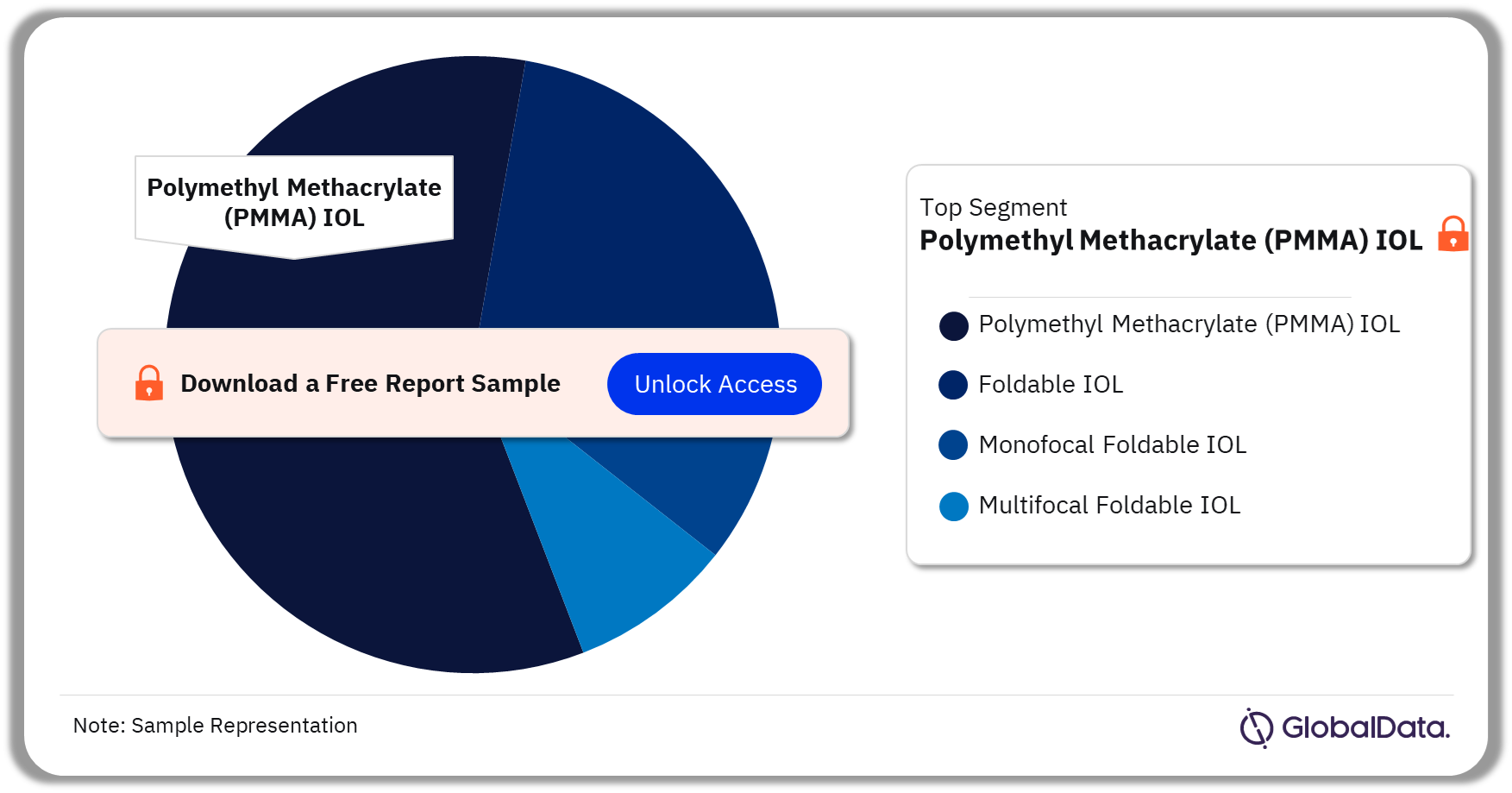
The key segments in the IOL market are Polymethyl Methacrylate (PMMA) IOL, Foldable IOL, Monofocal Foldable IOL, and Multifocal Foldable IOL. As of July 2023, Polymethyl Methacrylate (PMMA) IOL has the highest number of pipeline products. Such IOLs remain a preferred choice for people with a history of uveitis, diabetic retinopathy requiring vitrectomy, or at high risk of retinal detachment.
IOL Pipeline Market Analysis by Segments, 2023 (%)
For more segment insights into the IOL pipeline market, download a free report sample
IOL Pipeline Market Segmentation by Territories
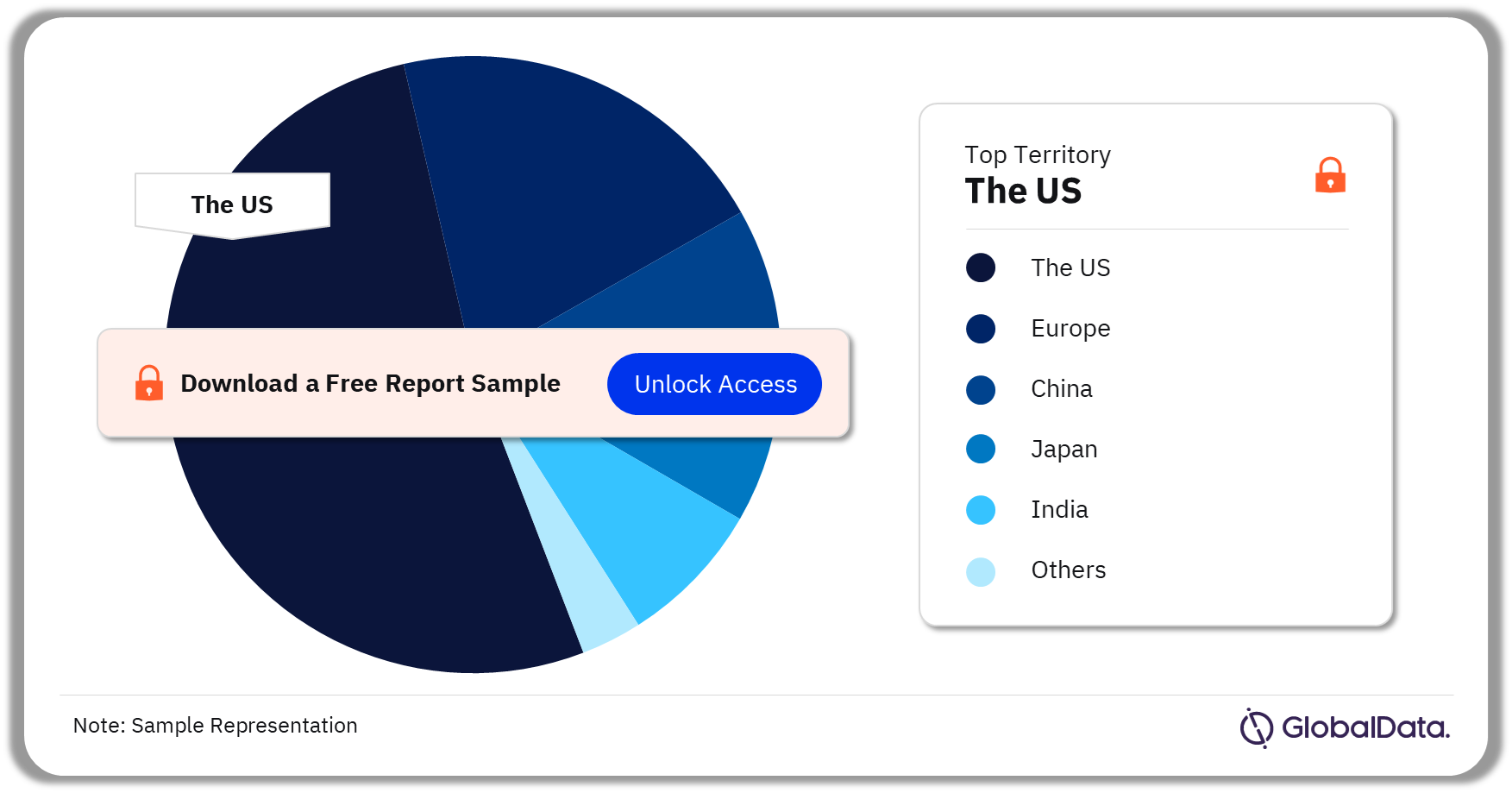
Some of the key territories in the IOL market are the US, Europe, China, Japan, India, Canada, Australia, New Zealand, Singapore, and Israel. As of July 2023, the US has the highest number of pipeline products.
IOL Pipeline Market Analysis by Territories, 2023 (%)
For more territory insights into the IOL pipeline market, download a free report sample
IOL Pipeline Market Segmentation by Regulatory Paths
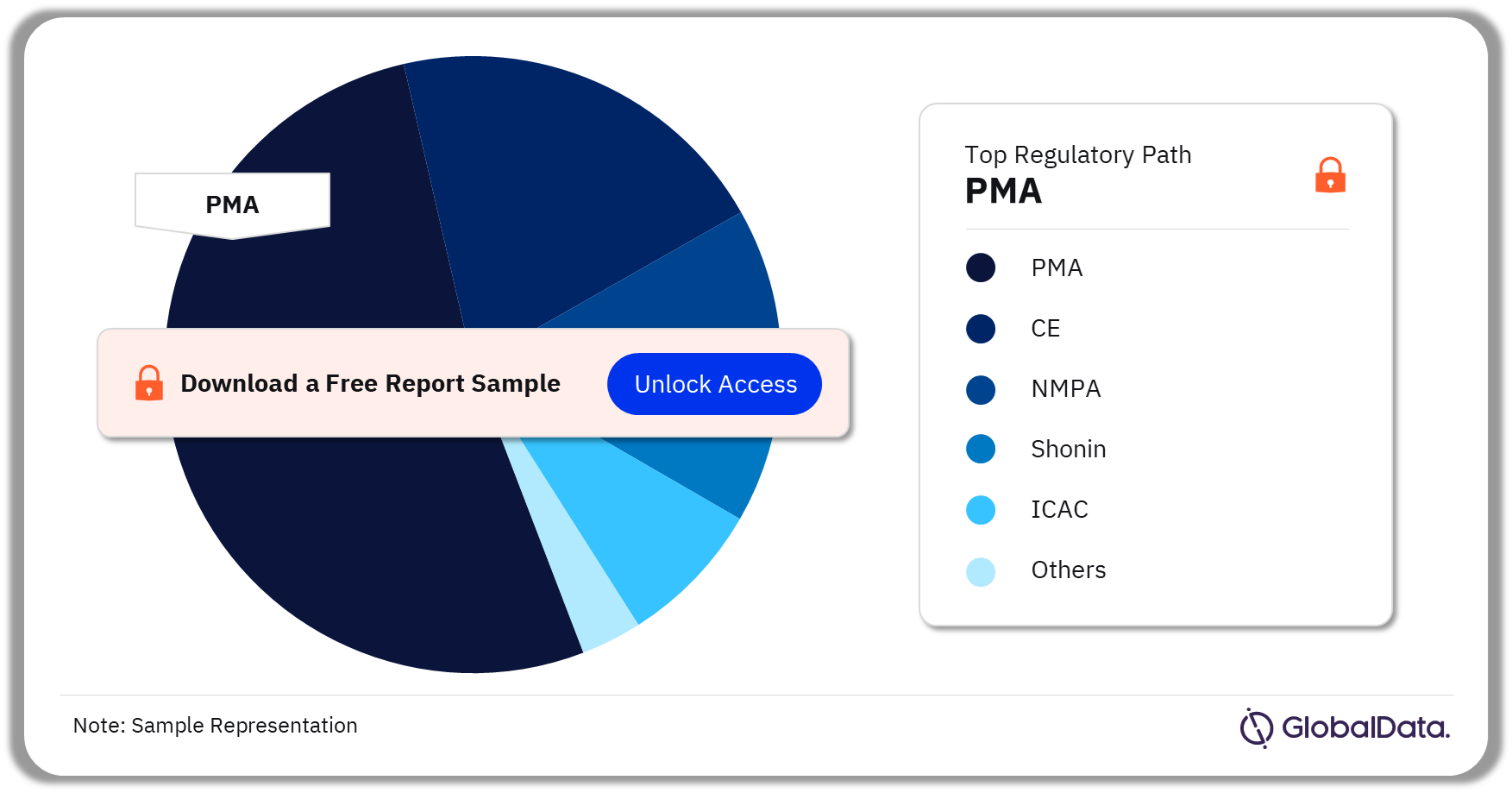
Some of the key regulatory paths in the IOL pipeline market are PMA, CE, NMPA, Shonin, ICAC, MDL, HDE Approvals, HSA, TGA, and 510(k). As of July 2023, PMA is the most followed pathway for pipeline products in the market.
IOL Pipeline Market Analysis by Regulatory Paths, 2023 (%)
For more regulatory path insights into the IOL pipeline market, download a free report sample
IOL Pipeline Market – Competitive Landscape
Some of the key companies in the IOL pipeline market are Adaptilens LLC, Adoptics AG (Inactive), AkkoLens International BV, Alcon Inc, Anew Optics, Inc., Atia Vision Inc, Bausch & Lomb Inc, Care Group, CEYEBER Corp, and Clarvista Medical Inc.
AkkoLens International BV: The medical device company provides products such as intraocular lenses and ophthalmological surgery equipment. It offers clinical trials, registration, and sales and investment services. AkkoLens’ products are used for therapeutic areas of cataract and reading-far-sightedness. The company is headquartered in Breda, the Netherlands.
Alcon Inc: The company is engaged in the development and manufacturing of devices in the field of ophthalmology. The company’s portfolio encompasses contact lenses and surgical products, including implantables, consumables, and surgical equipment. The company’s products are indicated for the treatment of various conditions such as cataracts, glaucoma, retinal diseases, and refractive errors. Alcon is headquartered in Geneva, Switzerland.
Leading Intraocular Lens (IOL) Companies, 2023
For more company insights into the IOL pipeline market, download a free report sample
Segments Covered in the Report
IOL Pipeline Market Segments Outlook
- Polymethyl Methacrylate (PMMA) IOL
- Foldable IOL
- Monofocal Foldable IOL
- Multifocal Foldable IOL
IOL Pipeline Market Territories Outlook
- The US
- Europe
- China
- Japan
- India
- Canada
- Australia
- New Zealand
- Singapore
- Israel
IOL Pipeline Market Regulatory Paths Outlook
- PMA
- CE
- NMPA
- Shonin
- ICAC
- MDL
- HDE Approvals
- HAS
- TGA
- 510(k)
Scope
This report provides:
- Extensive coverage of the Intraocular Lens (IOL) under development.
- Details of major pipeline products which includes product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Intraocular Lens (IOL) and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of IOL under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Adoptics AG (Inactive)
AkkoLens International BV
Alcon Inc
Anew Optics, Inc.
Atia Vision Inc
Bausch & Lomb Inc
Care Group
CEYEBER Corp
Clarvista Medical Inc
ClearSight LLC
Cord LLC
Elenza, Inc.
Emmetrope Incorporated
EyeKon Medical Inc
ForSight Vision6 Inc
Gaush Meditech Ltd
Henan Universe Intraocular Lens Development Co Ltd
Hoya Surgical Optics Inc
Innovia LLC
Johnson & Johnson Surgical Vision Inc
LensGen Inc
London Eye Hospital
Medicontur Medical Engineering Ltd
Meir Medical Center
Morcher Gmbh
Nayam Innovations Pvt Ltd
Nidek Co Ltd
Northwestern University
NuLens Ltd. (Inactive)
Ocumetics Technology Corp
PhysIOL SA
PowerVision Inc
Presbeasy
Rayner Intraocular Lenses Ltd
Sav-Iol SA
Shanghai Haohai Biological Technology Co Ltd
STAAR Surgical Company
Strathspey Crown LLC
Tianjin Century Kangtai Biomedical Engineering Co Ltd
Ulsan National Institute of Science and Technology
University of East Anglia
University of Rochester
University of Tampere
VA St. Louis Health Care System
Visidome Ltd
Visioneering Technologies Inc
VisusNano Labs
Voptica SL
VSY Biotechnology BV
Washington University in St Louis
Xend
Xi'an Eyedele Medical Technology Co Ltd
Z Lens LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the Intraocular Lens (IOL) pipeline market?
Polymethyl Methacrylate (PMMA) IOL is the leading segment in the IOL pipeline market.
-
Which territory has the highest number of pipeline products in the Intraocular Lens (IOL) pipeline market?
As of July 2023, the US has the highest number of pipeline products in the IOL pipeline market.
-
Which is the most followed regulatory pathway in the Intraocular Lens (IOL) pipeline market?
As of July 2023, PMA is the most followed pathway for pipeline products in the IOL pipeline market.
-
Who are the major companies operating in the Intraocular Lens (IOL) pipeline market?
Some of the key companies in the IOL pipeline market are Adaptilens LLC, Adoptics AG (Inactive), AkkoLens International BV, Alcon Inc, Anew Optics, Inc., Atia Vision Inc, Bausch & Lomb Inc, Care Group, CEYEBER Corp, and Clarvista Medical Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Intraocular Lens (IOL) reports