Interbody Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Interbody Devices Pipeline Market Report Overview
Interbody fusion cage devices use hollow threaded titanium or carbon fiber cylinder. It is used to fuse two vertebrae to treat degenerative disc disease to provide fixation. During the surgery process, the diseased/ruptured vertebral disc is replaced by interbody cages.
Interbody devices are made up of materials such as metals usually titanium, machined bone allograft, PEEK (polyetheretherketone), and others which include ceramics and carbon fiber. Depending upon the surgical approach followed, thoracolumbar spinal procedures involving interbody devices are divided into four types namely Anterior lumbar interbody fusion (ALIF), Posterior lumbar interbody fusion (PLIF), Transforaminal lumbar interbody fusion (TLIF) and Lateral lumbar interbody fusion (LLIF).
The interbody devices pipeline market research report provides comprehensive information about the Interbody Devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · PEEK Interbody Devices
· Metal Interbody Devices · Other Interbody Devices (Ceramic, Carbon Fibre) · Machined Allograft Bone Interbody Devices |
| Key Territories | · The US
· Europe · China · Australia · United Kingdom |
| Key Regulatory Paths | · 510(k)
· PMA · CE · NMPA · TGA |
| Leading Companies | · 3D Systems Inc
· Acuitive Technologies Inc · Allegra Orthopaedics Ltd · ALM Ortho Inc · Alphatec Holdings Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Interbody Devices Pipeline Market by Segments
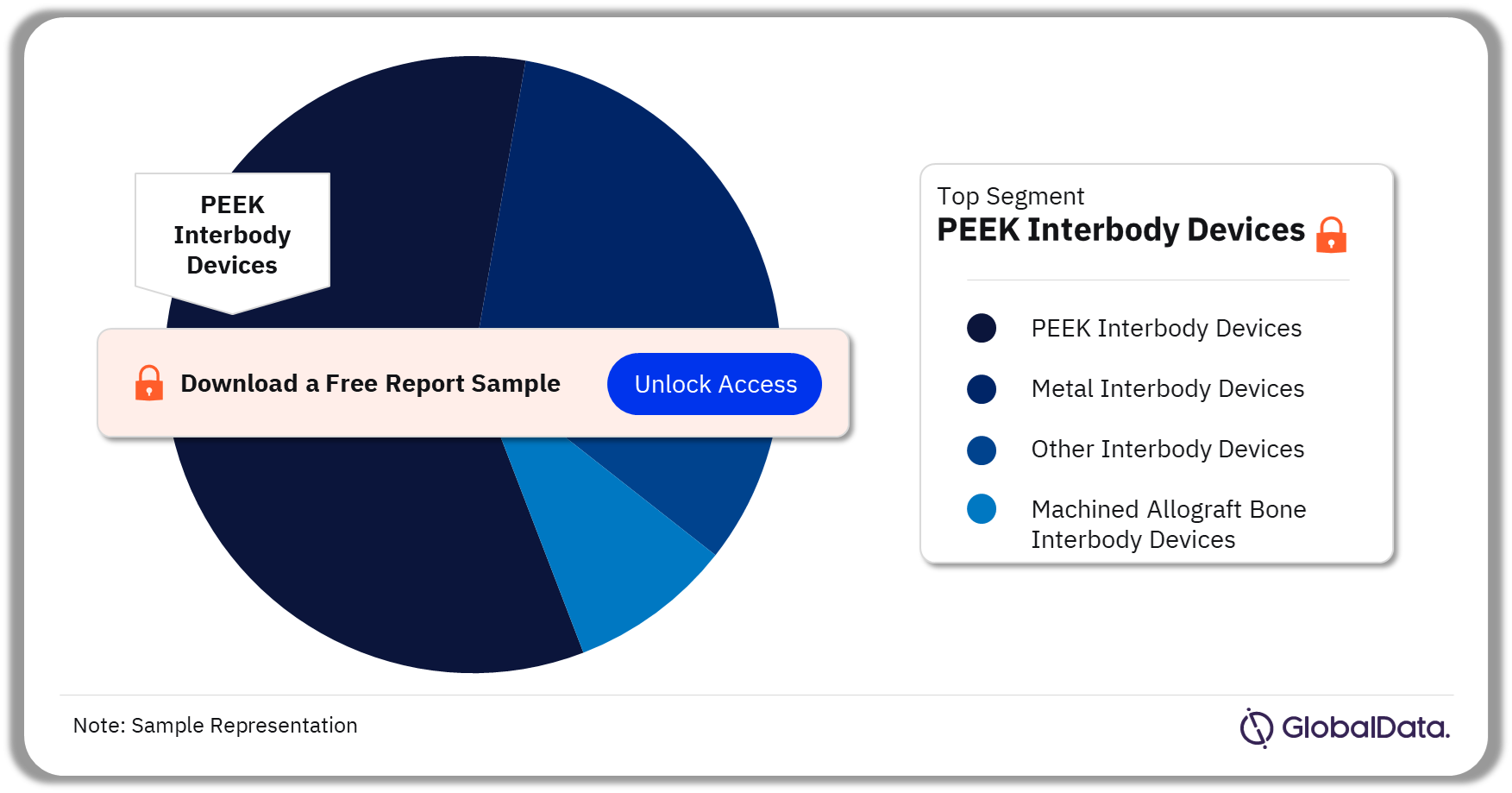
The key segments in the interbody devices market are PEEK Interbody Devices, Metal Interbody Devices, Other Interbody Devices (Ceramic, Carbon Fibre), and Machined Allograft Bone Interbody Devices. As of July 2023, the PEEK Interbody Devices segment had the highest number of pipeline products.
Interbody Devices Pipeline Market Analysis by Segments, 2023 (%)
For more segment insights into the Interbody Devices pipeline market, download a free report sample
Interbody Devices Pipeline Market Segmentation by Territories
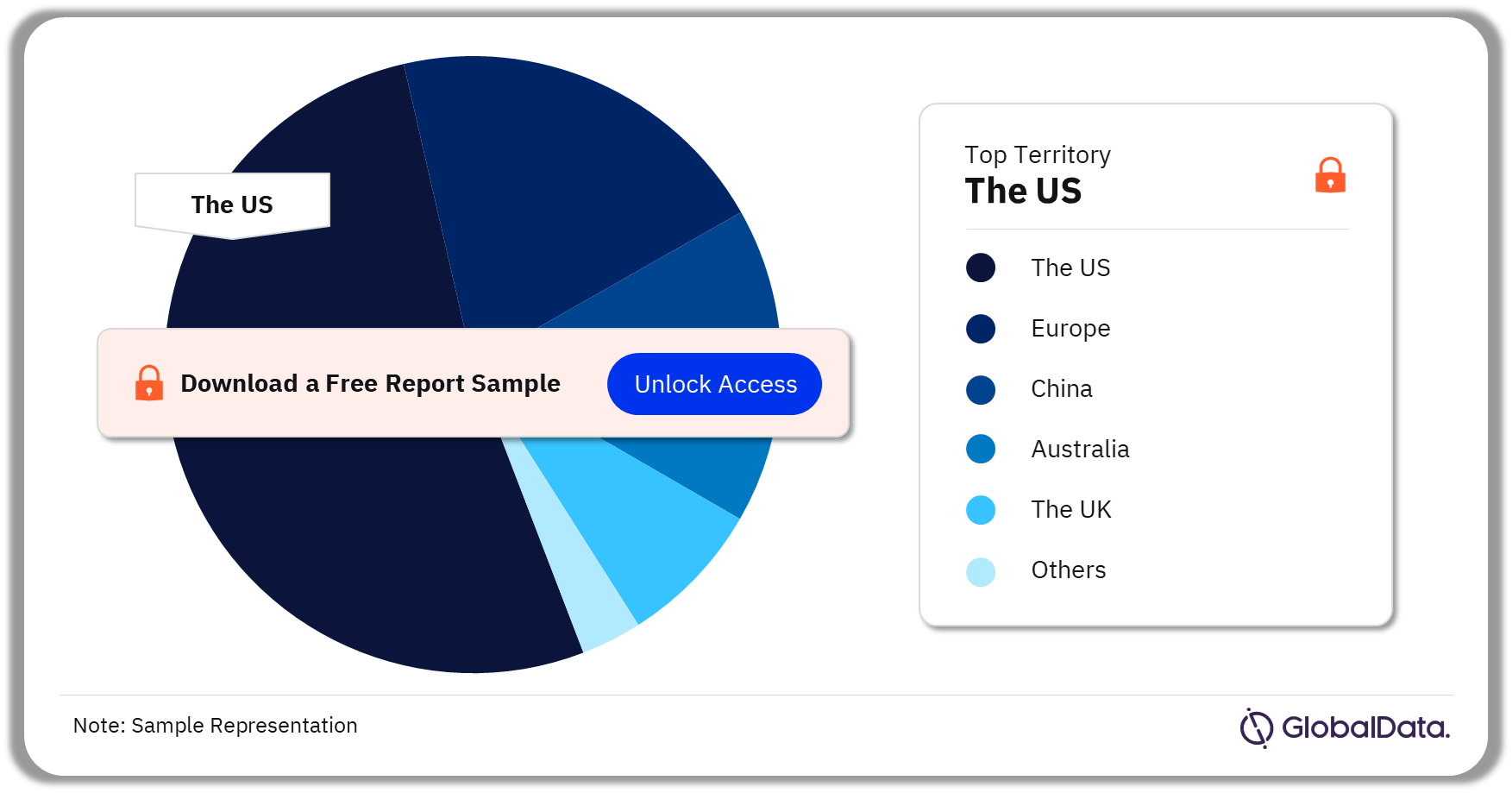
Some of the key territories in the Interbody Devices market are the US, Europe, China, Australia, and the United Kingdom among others. As of July 2023, the US had the highest number of pipeline products.
Interbody Devices Pipeline Market Analysis by Territories, 2023 (%)
For more territory insights into the Interbody Devices pipeline market, download a free report sample
Interbody Devices Pipeline Market Segmentation by Regulatory Paths
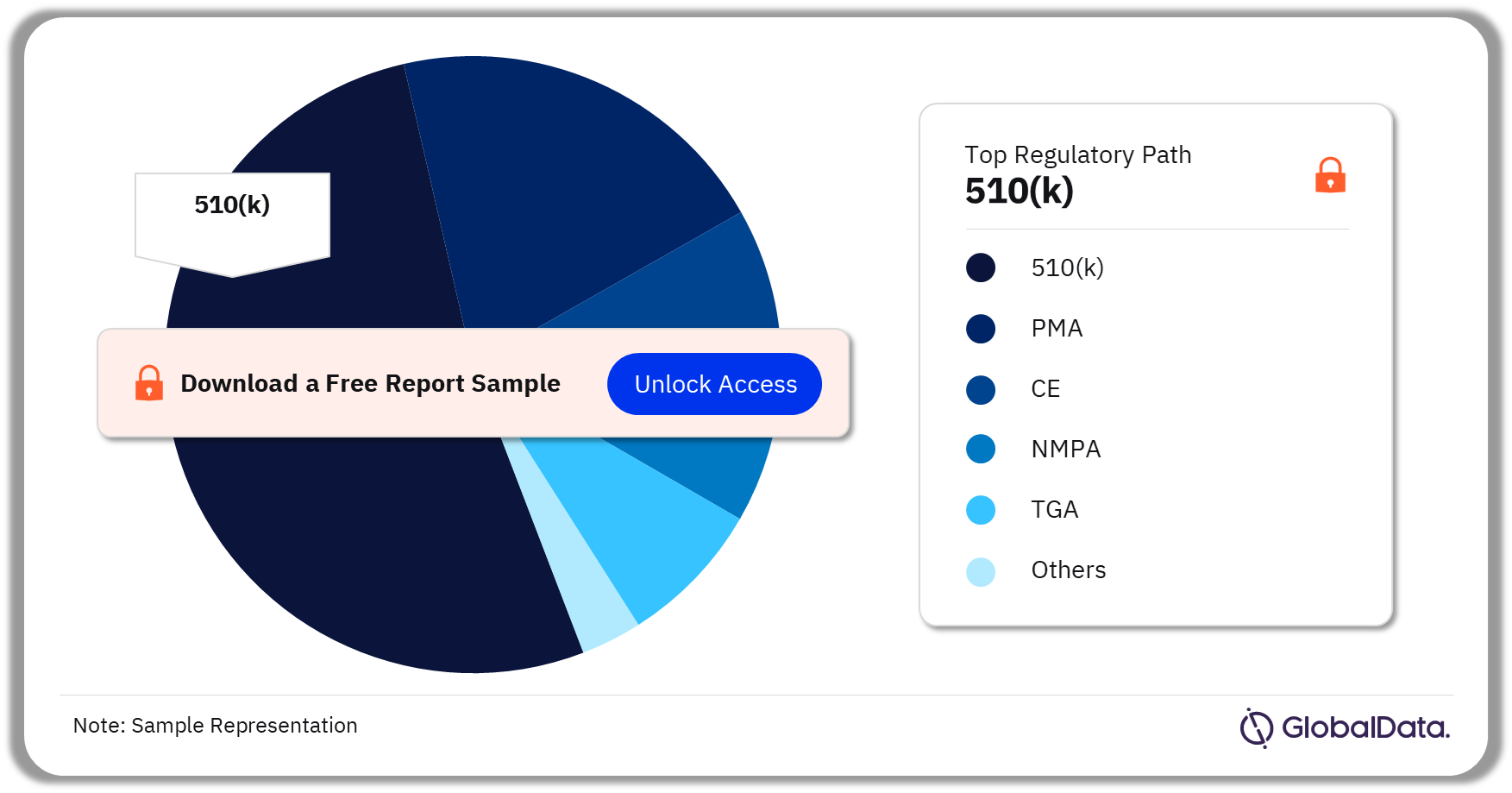
Some of the key regulatory paths in the Interbody Devices pipeline market are 510(k), PMA, CE, NMPA, and TGA among others. As of July 2023, 510(k) was the most followed pathway for pipeline products.
Interbody Devices Pipeline Market Analysis by Regulatory Paths, 2023 (%)
For more regulatory path insights into the Interbody Devices pipeline market, download a free report sample
Interbody Devices Pipeline Market – Competitive Landscape
Some of the key companies in the Interbody Devices pipeline market are 3D Systems Inc, Acuitive Technologies Inc, Allegra Orthopaedics Ltd, ALM Ortho Inc, and Alphatec Holdings Inc among others.
Allegra Orthopaedics Ltd Company: Allegra Orthopaedics Ltd (Allegra), develops, manufactures, and sells prosthetic implants and related orthopedic medical devices. Its products include adult and adolescent clavicle fixation systems and pins, shoulder implants, proximal humeral plates, and splints among others. Allegra also provides integrated services to surgeons and hospitals. It offers manufacturing services such as instrument manufacturing and precision manufacturing to other companies. The company’s operations are predominantly in Australia. Allegra is headquartered in Lane Cove West, New South Wales, Australia.
Leading Interbody Devices Companies, 2023
For more company insights into the Interbody Devices pipeline market, download a free report sample
Segments Covered in the Report
Interbody Devices Pipeline Market Segments Outlook
- PEEK Interbody Devices
- Metal Interbody Devices
- Other Interbody Devices (Ceramic, Carbon Fibre)
- Machined Allograft Bone Interbody Devices
Interbody Devices Pipeline Market Territories Outlook
- The US
- Europe
- China
- Australia
- United Kingdom
Interbody Devices Pipeline Market Regulatory Paths Outlook
- 510(k)
- PMA
- CE
- NMPA
- TGA
Scope
This report provides:
- Extensive coverage of the Interbody Devices under development.
- Details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Interbody Devices and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Interbody Devices under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Acuitive Technologies Inc
Allegra Orthopaedics Ltd
ALM Ortho Inc
Alphatec Holdings Inc
Biedermann Motech GmbH & Co KG
Bio2 Technologies Inc
Camber Spine Technologies LLC
Choice Spine LLC
Corelink LLC
CTL Amedica Corp
Cutting Edge Spine LLC
DePuy Synthes Inc
DSM Biomedical BV
Eminent Spine LLC
Evoke Medical LLC
Farmove Medical
Fuse Medical Inc
HAPPE Spine LLC
HD LifeSciences LLC
Innov'spine
Intelligent Implant Systems LLC
Jemo Spine, LLC.
LESspine LLC
Novum Medical Products, Inc.
Ozop Surgical Corp (Inactive)
Pharmaco-Kinesis Corp
SeaSpine, Inc.
SINTX Technologies Inc
Spinal Simplicity LLC
SpinalVu Inc (Inactive)
SpineSmith Holdings LLC
Stryker Corp
Taragenyx Ltd (Inactive)
Theradaptive Inc
TranS1 Inc
University of Pittsburgh
University of South Florida
VGI Medical LLC
Xtant Medical Holdings Inc
Xtremity & Spinal Solutions Ltd
Yunyi (Beijing) Medical Device Co Ltd
ZygoFix Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the interbody devices pipeline market?
PEEK Interbody Devices was the leading segment in the interbody devices pipeline market.
-
Which territory has the highest number of pipeline products in the interbody devices pipeline market?
As of July 2023, the US had the highest number of pipeline products in the interbody devices pipeline market.
-
Which was the most followed regulatory pathway in the interbody devices pipeline market?
As of July 2023, 510(k) was the most followed regulatory pathway in the interbody devices pipeline market.
-
Who are the major players operating in the interbody devices pipeline market?
Some of the key companies in the Interbody Devices pipeline market are 3D Systems Inc, Acuitive Technologies Inc, Allegra Orthopaedics Ltd, ALM Ortho Inc, and Alphatec Holdings Inc among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Interbody Devices reports