Hydrocephalus Shunts Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Hydrocephalus Shunts Pipeline Products Market Overview
Shunts allow excess cerebrospinal fluid (CSF) to drain to another area of the body. The Hydrocephalus Shunts pipeline market research report provides comprehensive information about the Hydrocephalus Shunts pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Hydrocephalus Shunts Pipeline Products Market by Segments
The key segment in the Hydrocephalus Shunts pipeline products market is Hydrocephalus Catheters.
Hydrocephalus Shunts Pipeline Products Market Segmentation by Territories
The key territories with products in the pipeline are the US, Europe, Argentina, Israel, and Japan, among others. As of April 2022, the US has the highest number of products in the pipeline out of them all.
Hydrocephalus Shunts Pipeline Products Market Analysis, by Territories
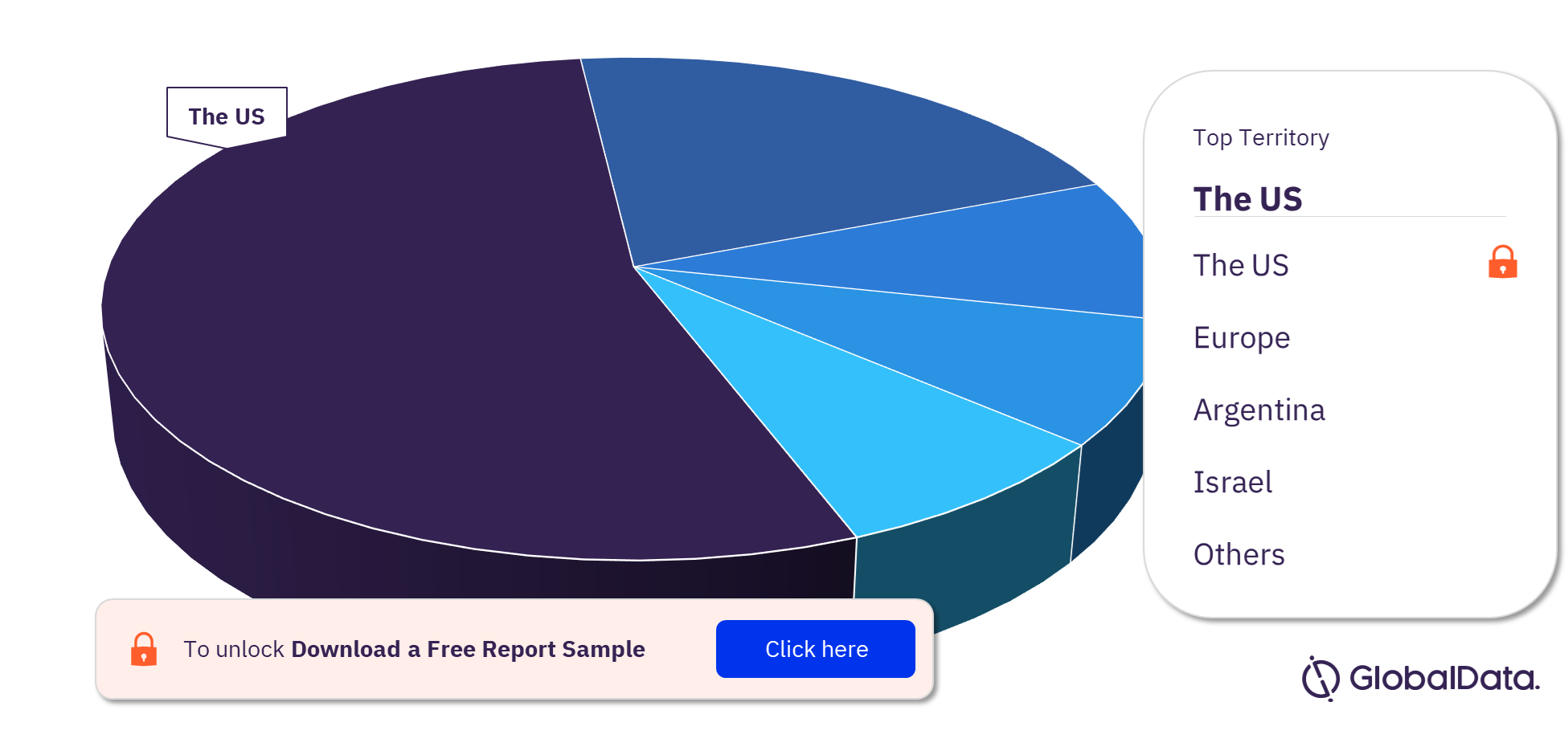 For more territory insights into the Hydrocephalus Shunts pipeline products market, download a free report sample
For more territory insights into the Hydrocephalus Shunts pipeline products market, download a free report sample
Hydrocephalus Shunts Pipeline Products Market Segmentation by Regulatory Paths
The key regulatory paths followed by the Hydrocephalus Shunts pipeline products market are 510(k), CE, and Ninsho. Most of the products follow the 510(k) pathway to enter the market.
Hydrocephalus Shunts Pipeline Products Market Analysis, by Regulatory Paths
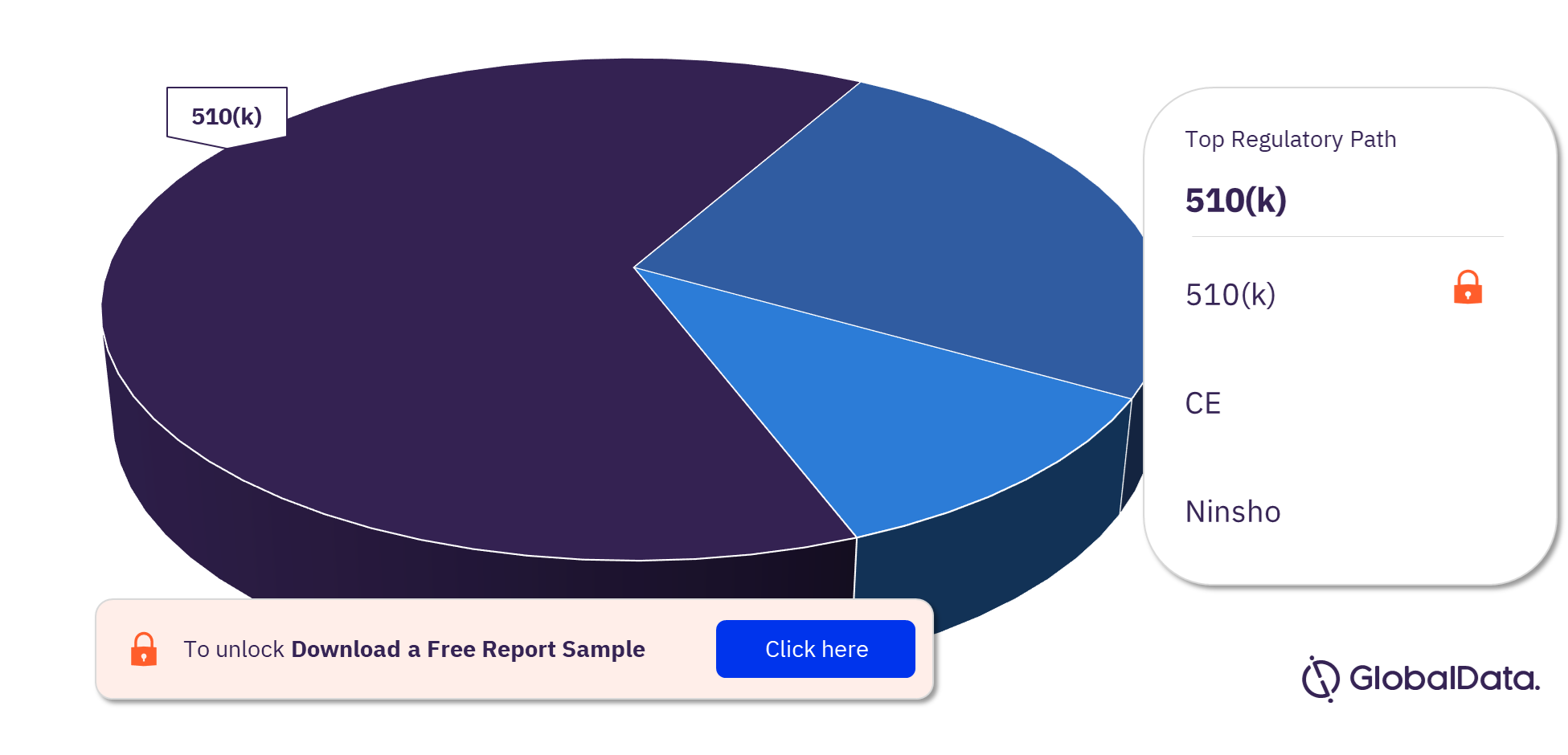 For more Hydrocephalus Shunts pipeline products regulatory path insights, download a free report sample
For more Hydrocephalus Shunts pipeline products regulatory path insights, download a free report sample
Hydrocephalus Shunts Pipeline Products Market - Competitive Landscape
Some of the leading companies in the Hydrocephalus Shunts pipeline products market are Anuncia Inc, Arkis BioSciences, Beckersmith Medical, Inc., Biosan Medical Ltd, CereVasc LLC, CSF-Dynamics AS, FreeFlow Medical Devices LLC, Georgia Institute of Technology, H-Cubed Inc., and Integra LifeSciences Holdings Corp.
Biosan Medical Ltd.: It is a medical device company that develops patented surgical devices. It also provides methods for the treatment of stroke and intraventricular hemorrhages of different etiologies. Biosan Medical is headquartered in Rishon Le’Zion, Israel.
Georgia Institute of Technology: It operates as a research university that offers undergraduate, graduate and research programs. The university provides various bachelor’s degrees, master’s degrees, and doctoral degree programs. Georgia Tech also offers research services in the areas of software engineering, artificial intelligence and machine learning, cybersecurity, and risk management among others. The university offers outstanding programs in business, design, liberal arts, and sciences. It operates in China, France, and China. Georgia Tech is headquartered in Atlanta, Georgia, the US.
Integra LifeSciences Holdings Corp: It is a medical technology company that offers engineered collagen-based product lines. Integra’s products find application in orthopedic extremity surgery, neurosurgery, and reconstructive and general surgery. Integra serves hospitals, outpatient surgery centers, physicians, veterinarian, and dental practices. The company markets its solutions through direct sales representatives and an extensive network of distributors. Integra is headquartered in Plainsboro, New Jersey, the US.
Hydrocephalus Shunts Pipeline Products Market Report Overview
| Key Segments | Hydrocephalus Catheters |
| Key Territories | The US, Europe, Argentina, Israel, and Japan |
| Key Regulatory Paths | 510(k), CE, and Ninsho |
| Leading Companies | Anuncia Inc, Arkis BioSciences, Beckersmith Medical, Inc., Biosan Medical Ltd, CereVasc LLC, CSF-Dynamics AS, FreeFlow Medical Devices LLC, Georgia Institute of Technology, H-Cubed Inc., and Integra LifeSciences Holdings Corp |
Scope
This report provides:
- Extensive coverage of the Hydrocephalus Shunts under development.
- Reviews of details of major pipeline products which includes product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Hydrocephalus Shunts and lists all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from early development to the approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with a potentially strong product portfolio and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Hydrocephalus Shunts under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Arkis BioSciences
Beckersmith Medical, Inc.
Biosan Medical Ltd
CereVasc LLC
CSF-Dynamics AS
FreeFlow Medical Devices LLC
Georgia Institute of Technology
H-Cubed Inc.
Integra LifeSciences Holdings Corp
Johns Hopkins University
Microbot Medical Ltd
Monitor Med Solutions
NeuraMedica, LLC
Sree Chitra Tirunal Institute for Medical Sciences & Technology
System Science Inc.
University Hospital Zurich
University of California Los Angeles
University of Iowa
University of Rochester
University of South Florida
University of Wisconsin Madison
Vivonics Inc
Wayne State University
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key segments in the Hydrocephalus Shunts pipeline products market?
The key segment in the Hydrocephalus Shunts pipeline products market is Hydrocephalus Catheters.
-
What are the key territories in the Hydrocephalus Shunts pipeline products market?
The US, Europe, Argentina, Israel, and Japan are some of the key territories with products in the pipeline.
-
What are the key regulatory paths of the Hydrocephalus Shunts pipeline products market?
The key regulatory paths followed by the Hydrocephalus Shunts pipeline products market are 510(k), CE, and Ninsho.
-
What are the leading companies in the Hydrocephalus Shunts pipeline products market?
Some of the leading companies in the Hydrocephalus Shunts pipeline products market are Anuncia Inc, Arkis BioSciences, Beckersmith Medical, Inc., Biosan Medical Ltd, CereVasc LLC, CSF-Dynamics AS, FreeFlow Medical Devices LLC, Georgia Institute of Technology, H-Cubed Inc., and Integra LifeSciences Holdings Corp.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Neurology Devices reports









