Injectable Drug Delivery – Pipeline Products by Stage of Development 32
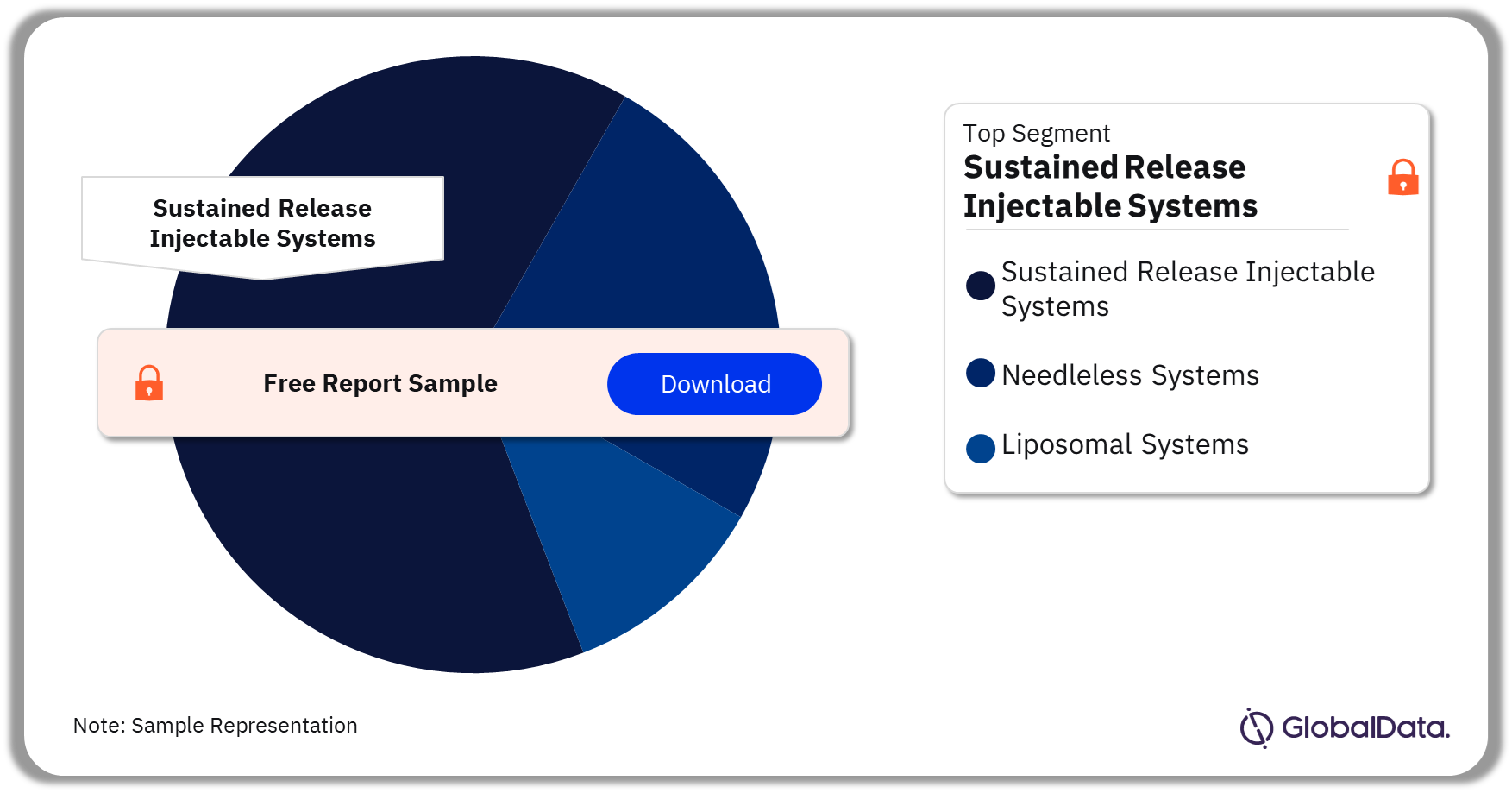
Injectable Drug Delivery – Pipeline Products by Segment 33
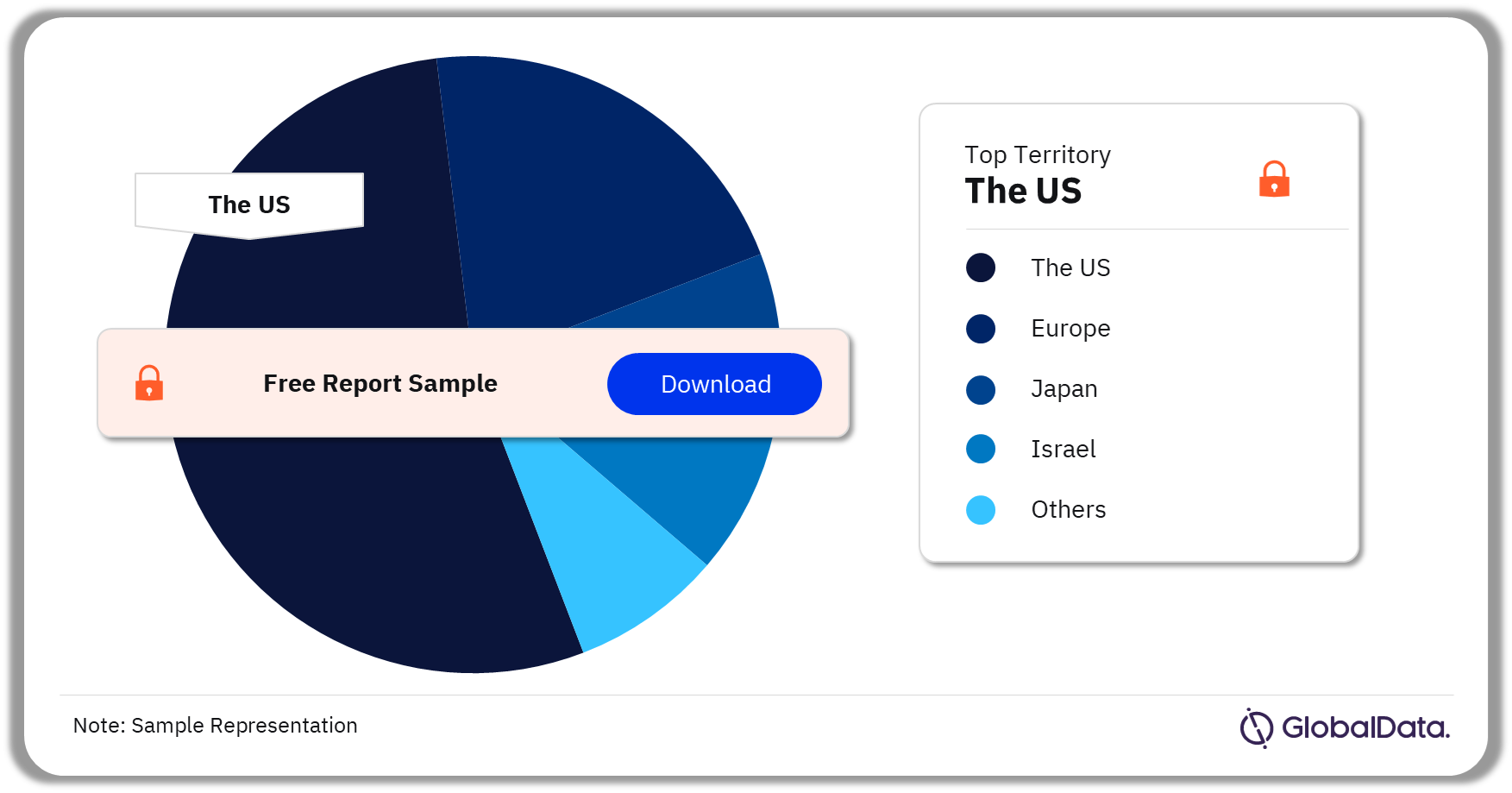
Injectable Drug Delivery – Pipeline Products by Territory 34
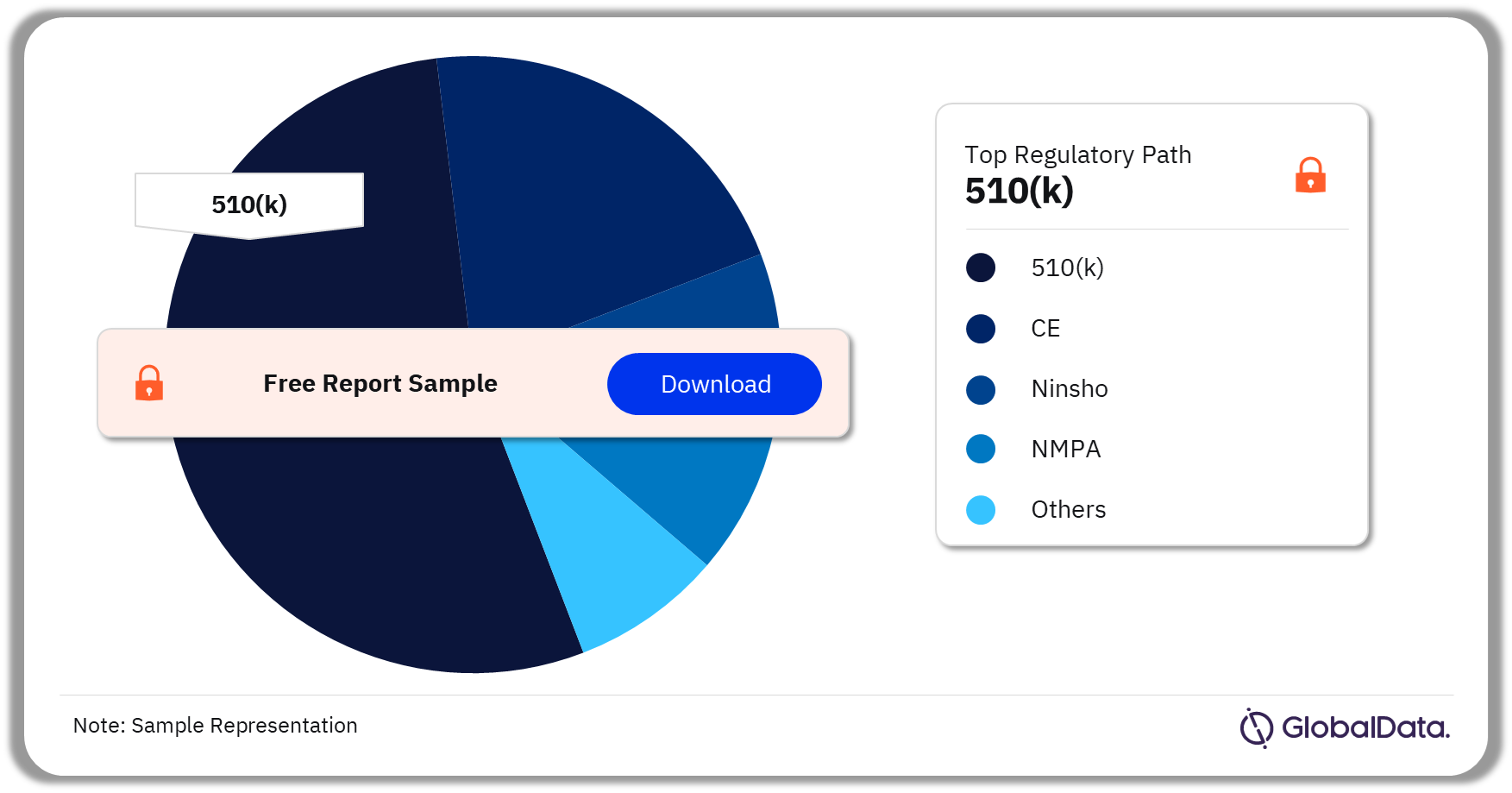
Injectable Drug Delivery – Pipeline Products by Regulatory Path 36
Injectable Drug Delivery – Pipeline Products by Estimated Approval Date 37
Injectable Drug Delivery – Ongoing Clinical Trials 38
Injectable Drug Delivery Companies – Pipeline Products by Stage of Development 39
Injectable Drug Delivery – Pipeline Products by Stage of Development 46
AcelRx Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 53
PFS-01 – Product Status 53
PFS-01 – Product Description 53
PFS-02 – Product Status 54
PFS-02 – Product Description 54
Actuated Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 55
OSII – Product Status 55
OSII – Product Description 55
Adalvo Pipeline Products & Ongoing Clinical Trials Overview 56
Icatibant Pre-Filled Syringe – Product Status 56
Icatibant Pre-Filled Syringe – Product Description 56
AdrenaCard, Inc. Pipeline Products & Ongoing Clinical Trials Overview 57
Adrenacard Auto-Injector – Product Status 57
Adrenacard Auto-Injector – Product Description 57
AktiVax Inc. Pipeline Products & Ongoing Clinical Trials Overview 58
ARAI-2PAM – Product Status 58
ARAI-2PAM – Product Description 58
Alexion Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 59
ALXN1720 Prefilled Syringe – Product Status 59
ALXN1720 Prefilled Syringe – Product Description 59
Alexion Pharmaceuticals Inc – Ongoing Clinical Trials Overview 60
ALXN1720 Prefilled Syringe – A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel, Multicenter Study to Evaluate the Safety and Efficacy of ALXN1720 in Adults With Generalized Myasthenia Gravis 61
Allergan Ltd Pipeline Products & Ongoing Clinical Trials Overview 62
NOVADUR Intravitreal Implant – Product Status 62
NOVADUR Intravitreal Implant – Product Description 62
Alsensa ApS Pipeline Products & Ongoing Clinical Trials Overview 63
SQJCT – Product Status 63
SQJCT – Product Description 63
Alvotech Swiss AG Pipeline Products & Ongoing Clinical Trials Overview 64
AVT06 – Eylea – Product Status 64
AVT06 – Eylea – Product Description 64
Alvotech Swiss AG – Ongoing Clinical Trials Overview 65
AVT06 – Eylea – Single-arm, Open Label, Multicenter Clinical Study to Evaluate the Handling and Safety of AVT06 Pre-filled Syringe (PFS) in Subjects With Chorioretinal Vascular Disease Followed by an Optional Extension Phase of AVT06 Pre-filled Syringe (PFS) 66
Amneal Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 67
Dihydroergotamine Autoinjector – Product Status 67
Dihydroergotamine Autoinjector – Product Description 67
Antares Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 68
ATRS-1901 Auto-Injector – Product Status 68
ATRS-1901 Auto-Injector – Product Description 68
Methotrexate Auto Injector – Ectopic Pregnancy – Product Status 69
Methotrexate Auto Injector – Ectopic Pregnancy – Product Description 69
Rescue Pen – Product Status 69
Rescue Pen – Product Description 70
Selatogrel Auto-Injector – Product Status 70
Selatogrel Auto-Injector – Product Description 70
Teriparatide Disposable Pen Injector – Product Status 71
Teriparatide Disposable Pen Injector – Product Description 71
VAI ATRS-1902 Auto-Injector – Product Status 71
VAI ATRS-1902 Auto-Injector – Product Description 72
Vibex QS M Auto-Injector – Product Status 72
Vibex QS M Auto-Injector – Product Description 72
Antares Pharma Inc – Ongoing Clinical Trials Overview 73
Selatogrel Auto-Injector – Multi-center, Double-blind, Randomized, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Self-administered Subcutaneous Selatogrel for Prevention of All-cause Death and Treatment of Acute Myocardial Infarction in Subjects with a Recent History of Acute Myocardial Infarction 74
Apellis Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 75
Empaveli Injector – Product Status 75
Empaveli Injector – Product Description 76
Apellis Pharmaceuticals Inc – Ongoing Clinical Trials Overview 77
Empaveli Injector – An Open Label, Non-randomized, Multi-center Extension Study to Evaluate the Long-term Safety and Efficacy of Pegcetacoplan in the Treatment of Paroxysmal Nocturnal Hemoglobinuria (PNH) 78
ApiJect Systems Corp Pipeline Products & Ongoing Clinical Trials Overview 79
Prefilled ApiJect Injector – Future Version – Product Status 79
Prefilled ApiJect Injector – Future Version – Product Description 79
Prefilled ApiJect Injector – Three-part – Product Status 80
Prefilled ApiJect Injector – Three-part – Product Description 80
Prefilled ApiJect Injector – Two-Part – Product Status 80
Prefilled ApiJect Injector – Two-Part – Product Description 81
Aptissen SA Pipeline Products & Ongoing Clinical Trials Overview 82
EVI01 – Product Status 82
EVI01 – Product Description 82
TID01 – Product Status 83
TID01 – Product Description 83
Aquavit Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 84
Apollo (PITO-001) – Product Status 84
Apollo (PITO-001) – Product Description 84
Artemes Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 85
Artemes Magnetic Injection System – Product Status 85
Artemes Magnetic Injection System – Product Description 85
Ascendis Pharma AS Pipeline Products & Ongoing Clinical Trials Overview 86
TransCon hGH Auto-Injector – Product Status 86
TransCon hGH Auto-Injector – Product Description 87
TransCon PTH Pen Injector – Product Status 87
TransCon PTH Pen Injector – Product Description 88
Ascendis Pharma AS – Ongoing Clinical Trials Overview 89
TransCon hGH Auto-Injector – The Efficacy, Safety and Tolerability of TransCon hGH Administered Weekly Versus Daily hGH in Prepubertal Children with Growth Hormone Deficiency: a Randomized, Open-lable, Active-controlled, Parallel-group Study in China 90
TransCon PTH Pen Injector – A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-controlled, Parallel Group Trial, with an Open-Label Extension, Investigating the Safety, Tolerability and Efficacy of TransCon PTH Administered Subcutaneously Daily in Adults with Hypoparathyroidism: PaTHway TRIAL 91
TransCon PTH Pen Injector – PaTH Forward: A Phase II, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel Group Trial with an Open-label Extension, Investigating the Safety, Tolerability and Efficacy of TransCon PTH Administered Subcutaneously Daily in Adults with Hypoparathyroidism 91
ASCIL Biopharm Pipeline Products & Ongoing Clinical Trials Overview 92
ASTECH – Product Status 92
ASTECH – Product Description 92
Combo Connector – Dual Chamber Device – Product Status 93
Combo Connector – Dual Chamber Device – Product Description 93
DuoLapse – Product Status 93
DuoLapse – Product Description 93
Ash Access Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 94
Zuragen – Product Status 94
Zuragen – Product Description 94
ASIS Corp Pipeline Products & Ongoing Clinical Trials Overview 95
Automatic Subdermal Injection System – Product Status 95
Automatic Subdermal Injection System – Product Description 95
Auckland Bioengineering Institute Pipeline Products & Ongoing Clinical Trials Overview 96
Jet Injection – Product Status 96
Jet Injection – Product Description 96
AzureBio SL Pipeline Products & Ongoing Clinical Trials Overview 97
Injectable Needle – Product Status 97
Injectable Needle – Product Description 97
Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 98
BD Evolve On-body Injector – Product Status 98
BD Evolve On-body Injector – Product Description 98
BD Libertas Wearable Injector – Product Status 99
BD Libertas Wearable Injector – Product Description 99
BD Neopak Xtraflow – Product Status 99
BD Neopak Xtraflow – Product Description 99
Bespak Europe Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
INJ200 Autoinjector – Product Status 100
INJ200 Autoinjector – Product Description 100
Lila Mix Injector – Product Status 101
Lila Mix Injector – Product Description 101
Biocorp Production SA Pipeline Products & Ongoing Clinical Trials Overview 102
Injay – Product Status 102
Injay – Product Description 103
BioCorRx Inc Pipeline Products & Ongoing Clinical Trials Overview 104
Injectable Naltrexone Implant – BICX101 – Product Status 104
Injectable Naltrexone Implant – BICX101 – Product Description 105
Naltrexone Implant – BICX102 – Product Status 105
Naltrexone Implant – BICX102 – Product Description 105
Naltrexone Implant – BICX104 – Product Status 106
Naltrexone Implant – BICX104 – Product Description 106
BioCorRx Inc – Ongoing Clinical Trials Overview 107
Naltrexone Implant – BICX102 – Evaluate the Effectiveness of BioCorRx Recovery Program for Alcohol and/or Opioid Use Disorders 108
Biorchestra Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 109
RNA Drug Delivery System – Product Status 109
RNA Drug Delivery System – Product Description 109
BioRestorative Therapies Inc Pipeline Products & Ongoing Clinical Trials Overview 110
BRTX-100 – Product Status 110
BRTX-100 – Product Description 110
Curved Needle Device – Product Status 111
Curved Needle Device – Product Description 111
BioRestorative Therapies Inc – Ongoing Clinical Trials Overview 112
BRTX-100 – A Phase II, Double-blind, Saline-controlled, Randomized Study to Evaluate the Safety and Preliminary Efficacy of a Single Dose Intradiscal Injection of BRTX 100 for Patients with Chronic Lumbar Disc Disease (cLDD) 113
Bioxis Pharmaceuticals Pipeline Products & Ongoing Clinical Trials Overview 114
Vaccine Delivery Device – Product Status 114
Vaccine Delivery Device – Product Description 114
BoneSupport AB Pipeline Products & Ongoing Clinical Trials Overview 115
Cerament|G – Product Status 115
Cerament|G – Product Description 115
BoneSupport AB – Ongoing Clinical Trials Overview 116
Cerament|G – CERAMENT G Device Registry 117
Cerament|G – Evaluation of the Efficiency of the Bone Substitute Cerament-G Locally Delivering Gentamicin in the Treatment of Chronic Osteomyelitis of Long Bones: Randomized Multicentre Study in the CRIOAc Network – CONVICTION Study 117
Cerament|G – Two-stage Treatment of Large Bone Defects with Cerament G and Cerament V plus Auto and/or Allograft Using the Masquelet Technique as Salvage Therapy after Bone Resection in Patients with Septic Pseudarthrosis 117
Brigham and Women’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 118
i2T2 Device – Product Status 118
i2T2 Device – Product Description 118
Brixton Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 119
Coolio Therapy – Product Status 119
Coolio Therapy – Product Description 119
Burke Pharmaceuticals LLC Pipeline Products & Ongoing Clinical Trials Overview 120
BURKE201 Needle Injection Pen – Product Status 120
BURKE201 Needle Injection Pen – Product Description 120
Calla Health Foundation Pipeline Products & Ongoing Clinical Trials Overview 121
Automated Needle Injector – Product Status 121
Automated Needle Injector – Product Description 121
Cambridge Consultants Ltd Pipeline Products & Ongoing Clinical Trials Overview 122
Flexi-ject – Product Status 122
Flexi-ject – Product Description 122
Camurus AB Pipeline Products & Ongoing Clinical Trials Overview 123
CAM2029 Pre-Filled Syringe – Product Status 123
CAM2029 Pre-Filled Syringe – Product Description 124
CAM2032 Pre-Filled Syringe – Product Status 124
CAM2032 Pre-Filled Syringe – Product Description 124
CAM2038 Depot Injection – Product Status 125
CAM2038 Depot Injection – Product Description 125
CAM2043 Pre-Filled Injection – Product Status 125
CAM2043 Pre-Filled Injection – Product Description 126
CAM2047 Pre-Filled Syringe – Product Status 126
CAM2047 Pre-Filled Syringe – Product Description 126
Carmot Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 127
CT-868 Pen Injector – Product Status 127
CT-868 Pen Injector – Product Description 127
Carmot Therapeutics Inc – Ongoing Clinical Trials Overview 128
CT-868 Pen Injector – A Phase 2, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of CT-868 Administered for 16 Weeks to Overweight and Obese Adult Participants with Type 1 Diabetes Mellitus 129
Case Western Reserve University Pipeline Products & Ongoing Clinical Trials Overview 130
Pressure Driven Local Drug Delivery System – Liver Cancer – Product Status 130
Pressure Driven Local Drug Delivery System – Liver Cancer – Product Description 130
Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 131
Pre-Filled Syringe With Flush – Product Status 131
Pre-Filled Syringe With Flush – Product Description 131
Clearside BioMedical Inc Pipeline Products & Ongoing Clinical Trials Overview 132
SCS Microinjector – Product Status 132
SCS Microinjector – Product Description 133
Clearside BioMedical Inc – Ongoing Clinical Trials Overview 134
SCS Microinjector – A Phase 2 Open-label, Ascending Single and Repeat Dose Escalation Trial of Belzupacap Sarotalocan (AU-011) Via Suprachoroidal Administration in Subjects with Primary Indeterminate Lesions and Small Choroidal Melanoma 135
SCS Microinjector – A Phase 2, Randomized, Dose-escalation, Observation-controlled Study to Evaluate the Efficacy, Safety, and Tolerability of RGX-314 Gene Therapy Delivered via a Single Suprachoroidal Space (SCS) Injections in Participants With Diabetic Retinopathy (DR) Without Center Involved-diabetic Macular Edema (CI-DME): ALTITUDE 135
SCS Microinjector – A Phase 2, Randomized, Dose-escalation, Ranibizumab-controlled Study to Evaluate the Efficacy, Safety, and Tolerability of RGX-314 Gene Therapy Delivered Via One or Two Suprachoroidal Space (SCS) Injections in Participants with Neovascular Age-Related Macular Degeneration (nAMD): AAVIATE 135
SCS Microinjector – A Phase 3 Randomized, Masked, Controlled Trial to Evaluate Efficacy and Safety of Belzupacap Sarotalocan (AU-011) Treatment Compared to Sham Control in Subjects With Primary Indeterminate Lesions or Small Choroidal Melanoma 136
SCS Microinjector – ODYSSEY: A Phase 2b Study of Suprachoroidally Administered CLS-AX in Participants With Neovascular Age-Related Macular Degeneration 136
Coherus BioSciences Inc Pipeline Products & Ongoing Clinical Trials Overview 137
UDENYCA AI – Product Status 137
UDENYCA AI – Product Description 137
UDENYCA OBI – Product Status 138
UDENYCA OBI – Product Description 138
Copernicus Sp zoo Pipeline Products & Ongoing Clinical Trials Overview 139
PENDURA EVO – Product Status 139
PENDURA EVO – Product Description 139
CrossCoat Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 140
CCM-D – Product Status 140
CCM-D – Product Description 140
Crossject SA Pipeline Products & Ongoing Clinical Trials Overview 141
ZENEO Hydrocortisone Autoinjector – Product Status 141
ZENEO Hydrocortisone Autoinjector – Product Description 141
CSL Behring LLC Pipeline Products & Ongoing Clinical Trials Overview 142
Hizentra 50mL Prefilled Syringe – Product Status 142
Hizentra 50mL Prefilled Syringe – Product Description 142
Curer Inc Pipeline Products & Ongoing Clinical Trials Overview 143
Injectable Whitlockite-Based Cement – Product Status 143
Injectable Whitlockite-Based Cement – Product Description 143
DALI Medical Devices Ltd. Pipeline Products & Ongoing Clinical Trials Overview 144
SAN-DV – Product Status 144
SAN-DV – Product Description 144
SAN-DV Pro – Product Status 145
SAN-DV Pro – Product Description 145
SAN-In – Product Status 145
SAN-In – Product Description 146
SAN-L – Product Status 146
SAN-L – Product Description 146
SAN-P – Product Status 147
SAN-P – Product Description 147
Synnect – Product Status 147
Synnect – Product Description 148
DelSite, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 149
GelSure – Product Status 149
GelSure – Product Description 149
DelSiTech Ltd Pipeline Products & Ongoing Clinical Trials Overview 150
DST1308 – Product Status 150
DST1308 – Product Description 150
Injectable Depot – HIV Prevention – Product Status 151
Injectable Depot – HIV Prevention – Product Description 151
Injectable Depot – Pregnancy Prevention – Product Status 151
Injectable Depot – Pregnancy Prevention – Product Description 152
Long-Acting Controlled Release Therapy Device – Ophthalmology – Product Status 152
Long-Acting Controlled Release Therapy Device – Ophthalmology – Product Description 152
Department of Biomedical Engineering Columbia University Pipeline Products & Ongoing Clinical Trials Overview 153
AquaDrive – Product Status 153
AquaDrive – Product Description 153
Difinity Solutions Pipeline Products & Ongoing Clinical Trials Overview 154
NeedSafe – Product Status 154
NeedSafe – Product Description 154
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 155
Injectable Hydrogel – Product Status 155
Injectable Hydrogel – Product Description 155
Duoject Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 156
Maverick-Emergency Auto-Injector – Product Status 156
Maverick-Emergency Auto-Injector – Product Description 156
E3D Elcam Drug Delivery Devices Pipeline Products & Ongoing Clinical Trials Overview 157
Flexi-Q eMU-C – Product Status 157
Flexi-Q eMU-C – Product Description 157
Flexi-Q PFS – Product Status 158
Flexi-Q PFS – Product Description 158
Patch Pump (eLVD) – Product Status 158
Patch Pump (eLVD) – Product Description 159
Ebenroth BV Pipeline Products & Ongoing Clinical Trials Overview 160
EpiWatch 1.0 – Product Status 160
EpiWatch 1.0 – Product Description 160
EpiWatch 2.0 – Product Status 161
EpiWatch 2.0 – Product Description 161
Ecole Polytechnique Federale de Lausanne Pipeline Products & Ongoing Clinical Trials Overview 162
SPOT-RVC – Product Status 162
SPOT-RVC – Product Description 162
EmboMedics Inc Pipeline Products & Ongoing Clinical Trials Overview 163
Second Generation Injectable Microsphere – Product Status 163
Second Generation Injectable Microsphere – Product Description 163
Embryyo Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 164
Auto-injector – Product Status 164
Auto-injector – Product Description 164
Emergent BioSolutions Inc Pipeline Products & Ongoing Clinical Trials Overview 165
AP007 – Sustained Release Nalmefene Injectable Device – Product Status 165
AP007 – Sustained Release Nalmefene Injectable Device – Product Description 166
D4 Auto Injector – Product Status 166
D4 Auto Injector – Product Description 166
EGRD-001 – Product Status 167
EGRD-001 – Product Description 167
Emergard – Product Status 167
Emergard – Product Description 168
Trobigard – Product Status 168
Trobigard – Product Description 168
EP Global Communications Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 169
MEMS Trigger Point Needle Device – Product Status 169
MEMS Trigger Point Needle Device – Product Description 169
EpicentRx Inc Pipeline Products & Ongoing Clinical Trials Overview 170
eLoop Administration Device – Product Status 170
eLoop Administration Device – Product Description 170
EpicentRx Inc – Ongoing Clinical Trials Overview 171
eLoop Administration Device – REPLATINUM: A Phase 3, Controlled, Open-label, Global Randomized Study of RRx-001 Administered Sequentially With a Platinum Doublet or a Platinum Doublet in Third-Line or Beyond Small Cell Lung Cancer 172
Establishment Labs SA Pipeline Products & Ongoing Clinical Trials Overview 173
Motiva Injector – Product Status 173
Motiva Injector – Product Description 173
Eveon SAS Pipeline Products & Ongoing Clinical Trials Overview 174
Automated Injection Device – Product Status 174
Automated Injection Device – Product Description 174
Injection System – Product Status 175
Injection System – Product Description 175
EyeCool Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 176
ETX-4143 – Product Status 176
ETX-4143 – Product Description 176
EyeCool Therapeutics Inc – Ongoing Clinical Trials Overview 177
ETX-4143 – A First In Human Safety Study Of Etx-4143 (A Topical Cooling Device For The Eye) In Subjects With Chronic Eye Pain 178
EyePoint Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 179
Durasert – Front Of Eye Disease – Product Status 179
Durasert – Front Of Eye Disease – Product Description 180
Durasert – Knee Osteoarthritis – Product Status 180
Durasert – Knee Osteoarthritis – Product Description 180
Durasert – Latanoprost Implant – Product Status 181
Durasert – Latanoprost Implant – Product Description 181
EYP-1901 – Anti-VEGF Bioerodible Durasert – Product Status 181
EYP-1901 – Anti-VEGF Bioerodible Durasert – Product Description 182
Tethadur System – Product Status 182
Tethadur System – Product Description 182
YUTIQ 50 – Product Status 183
YUTIQ 50 – Product Description 183
EyePoint Pharmaceuticals Inc – Ongoing Clinical Trials Overview 184
EYP-1901 – Anti-VEGF Bioerodible Durasert – A Phase 2, Multicenter, Prospective, Double-masked, Parallel Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Sham for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy (NPDR) 185
EYP-1901 – Anti-VEGF Bioerodible Durasert – A Phase 2, Multicenter, Prospective, Randomized, Double-Masked, Parallel Study of EYP-1901, a Tyrosine Kinase Inhibitor (TKI), Compared to Aflibercept in Subjects with Wet AMD 185
F. Hoffmann-La Roche Ltd Pipeline Products & Ongoing Clinical Trials Overview 186
Susvimo Port Delivery System (PDS) – Product Status 186
Susvimo Port Delivery System (PDS) – Product Description 187
F. Hoffmann-La Roche Ltd – Ongoing Clinical Trials Overview 188
Susvimo Port Delivery System (PDS) – A Multicenter, Open-label Extension Study to Evaluate the Long-term Safety and Tolerability of the Port Delivery System with Ranibizumab in Patients with Neovascular Age-related Macular Degeneration (Portal) 189
Susvimo Port Delivery System (PDS) – A Phase III, Multicenter, Randomized, Visual Assessor-Masked, Active Comparator Study Of The Efficacy, Safety, And Pharmacokinetics Of The Port Delivery System With Ranibizumab In Chinese Patients With Neovascular Age-Related Macular Degeneration 189
Susvimo Port Delivery System (PDS) – A Phase IIIb/IV, Multicenter, Open-label, Single-arm Study of the Efficacy and Safety of the Port Delivery System with Ranibizumab In Patients with Neovascular Age-related Macular Degeneration Previously Treated with Intravitreal Agents Other than Ranibizumab 190
FHC Inc Pipeline Products & Ongoing Clinical Trials Overview 191
Intracerebral Microinjection Instrument – Product Status 191
Intracerebral Microinjection Instrument – Product Description 191
FluGen Inc Pipeline Products & Ongoing Clinical Trials Overview 192
Flugen 101.2 Microneedle-Based Delivery Device – Product Status 192
Flugen 101.2 Microneedle-Based Delivery Device – Product Description 192
Microneedle Based Delivery Device – Human Papillomavirus – Product Status 193
Microneedle Based Delivery Device – Human Papillomavirus – Product Description 193
Microneedle Delivery Device – Pneumococal – Product Status 193
Microneedle Delivery Device – Pneumococal – Product Description 194
Microneedle Delivery Device – Seasonal TIV – Product Status 194
Microneedle Delivery Device – Seasonal TIV – Product Description 194
Multivaccine Delivering Microneedle Based Device – Product Status 195
Multivaccine Delivering Microneedle Based Device – Product Description 195
Fresenius Kabi AG Pipeline Products & Ongoing Clinical Trials Overview 196
Pegfilgrastim – Pre-Filled Syringe – Product Status 196
Pegfilgrastim – Pre-Filled Syringe – Product Description 196
GeneSYS-ID Inc Pipeline Products & Ongoing Clinical Trials Overview 197
Rx MyDOSE – Product Status 197
Rx MyDOSE – Product Description 197
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 198
ePatch – Product Status 198
ePatch – Product Description 198
Injection Device – Eye Disease – Product Status 199
Injection Device – Eye Disease – Product Description 199
Georgia Institute of Technology – Ongoing Clinical Trials Overview 200
ePatch – Develop and Validate Machine-Learning Algorithm to Detect Atrial Fibrillation with Wearable Devices 201
ePatch – ECG Monitoring Short vs Middle Term After Atrial Fibrillation Ablation 201
Ghent University Pipeline Products & Ongoing Clinical Trials Overview 202
Poly-Electrolyte Micropartcle (PEM) System – Product Status 202
Poly-Electrolyte Micropartcle (PEM) System – Product Description 202
Globe Medical Tech Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 203
Dual Chamber Safety Prefillable Syringe – Product Status 203
Dual Chamber Safety Prefillable Syringe – Product Description 203
Single Chamber Safety Prefillable Syringe – Product Status 204
Single Chamber Safety Prefillable Syringe – Product Description 204
Halozyme Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 205
ENHANZE Plus Auto-Injector – Product Status 205
ENHANZE Plus Auto-Injector – Product Description 205
Hillmed Inc Pipeline Products & Ongoing Clinical Trials Overview 206
PelviMap System – Product Status 206
PelviMap System – Product Description 206
Hillmed Inc – Ongoing Clinical Trials Overview 207
PelviMap System – An Novel Medical System for Quantitative Diagnosis and Personalized Precision Botulinum Neurotoxin Injection in Chronic Pelvic Pain Management 208
Ichor Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 209
TriGrid ID – Product Status 209
TriGrid ID – Product Description 209
TriGrid IM – Product Status 210
TriGrid IM – Product Description 210
Ichor Medical Systems Inc – Ongoing Clinical Trials Overview 211
TriGrid IM – A Pilot Study to Assess the Safety and Immunogenicity of a Neoantigen-based Personalized DNA Vaccine With Retifanlimab PD-1 Blockade Therapy in Patients With Newly Diagnosed, Unmethylated Glioblastoma 212
Immunovant Inc Pipeline Products & Ongoing Clinical Trials Overview 213
IMVT-1401 – Product Status 213
IMVT-1401 – Product Description 213
Impulse Biomedical Pipeline Products & Ongoing Clinical Trials Overview 214
ZiBiPen – Product Status 214
ZiBiPen – Product Description 214
Inovio Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 215
Jupiter Jet – Product Status 215
Jupiter Jet – Product Description 215
InVivo Therapeutics Corp Pipeline Products & Ongoing Clinical Trials Overview 216
TrailMaker – Product Status 216
TrailMaker – Product Description 216
iScience Interventional Inc Pipeline Products & Ongoing Clinical Trials Overview 217
iScience Smart Needle – Product Status 217
iScience Smart Needle – Product Description 217
JCR Pharmaceuticals Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 218
Growject – SHOX Deficiency – Product Status 218
Growject – SHOX Deficiency – Product Description 218
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 219
MPACT-Needle – Product Status 219
MPACT-Needle – Product Description 220
LG Chem Ltd Pipeline Products & Ongoing Clinical Trials Overview 221
Y-Phasic – Product Status 221
Y-Phasic – Product Description 221
Liposphere Ltd Pipeline Products & Ongoing Clinical Trials Overview 222
AqueousJoint – Product Status 222
AqueousJoint – Product Description 222
Liposphere Ltd – Ongoing Clinical Trials Overview 223
AqueousJoint – Efficacy and Safety of AqueousJoint Intra-Articular Injections in Mild to Moderate Knee Osteoarthritis: Prospective, Placebo Controlled, Randomized, Double-Blinded Study 224
Love Lifesciences LLC Pipeline Products & Ongoing Clinical Trials Overview 225
UniPen – Product Status 225
UniPen – Product Description 225
LyoGo Pipeline Products & Ongoing Clinical Trials Overview 226
QuickStick – Product Status 226
QuickStick – Product Description 226
Mallinckrodt Plc Pipeline Products & Ongoing Clinical Trials Overview 227
Drug Delivery Device – Autoimmune Disorders – Product Status 227
Drug Delivery Device – Autoimmune Disorders – Product Description 227
Medovate Ltd Pipeline Products & Ongoing Clinical Trials Overview 228
Safira (SAFer Injection system for Regional Anaesthesia) – Product Status 228
Safira (SAFer Injection system for Regional Anaesthesia) – Product Description 228
Safira NRFit Syringe – Product Status 229
Safira NRFit Syringe – Product Description 229
Safira Palm Operator – Product Status 229
Safira Palm Operator – Product Description 230
Safira Foot Pedal – Product Status 230
Safira Foot Pedal – Product Description 230
Mercator MedSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 231
Bullfrog Micro-Infusion Device – Below The Knee – Product Status 231
Bullfrog Micro-Infusion Device – Below The Knee – Product Description 231
Bullfrog Micro-Infusion Device – Deep Vein Thrombosis – Product Status 232
Bullfrog Micro-Infusion Device – Deep Vein Thrombosis – Product Description 232
Mercator MedSystems Inc – Ongoing Clinical Trials Overview 233
Bullfrog Micro-Infusion Device – Below The Knee – Temsirolimus Adventitial Delivery to Improve Angiographic Outcomes below the Knee: TANGO 234
Bullfrog Micro-Infusion Device – Below The Knee – Temsirolimus Alone or Paired with Dexamethasone Delivered to the Adventitia to Enhance Clinical Efficacy after Femoropopliteal Revascularization 234
Bullfrog Micro-Infusion Device – Deep Vein Thrombosis – Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation after Thrombus Removal to Yield Benefit in Subacute and Chronic Iliofemoral DVT (DEXTERITY-SCI) 235
Mertiva AB Pipeline Products & Ongoing Clinical Trials Overview 236
Nerve Targeting Drug Delivery System – Product Status 236
Nerve Targeting Drug Delivery System – Product Description 236
Milestone Scientific Inc Pipeline Products & Ongoing Clinical Trials Overview 237
CompuFlo System – Cosmetic Surgery – Product Status 237
CompuFlo System – Cosmetic Surgery – Product Description 238
CompuFlo System – General Medical Injection – Product Status 238
CompuFlo System – General Medical Injection – Product Description 238
CompuFlo System – Ophthalmic Injection – Product Status 239
CompuFlo System – Ophthalmic Injection – Product Description 239
CompuFlo System – Peripheral Nerve Block – Product Status 239
CompuFlo System – Peripheral Nerve Block – Product Description 240
CompuFlo System – Self-Administered Drug Delivery – Product Status 240
CompuFlo System – Self-Administered Drug Delivery – Product Description 240
CompuFlo System – Thoracic – Product Status 241
CompuFlo System – Thoracic – Product Description 241
Mingche Biotechnology (Suzhou) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 242
Microneedle Drug Delivery Device – Product Status 242
Microneedle Drug Delivery Device – Product Description 242
Monash University Pipeline Products & Ongoing Clinical Trials Overview 243
Hydrogel-Based Delivery Device – Product Status 243
Hydrogel-Based Delivery Device – Product Description 243
Morimoto Pharma Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 244
S.A.F.E. Syringe Kit – Product Status 244
S.A.F.E. Syringe Kit – Product Description 244
NanoSyrinx Ltd Pipeline Products & Ongoing Clinical Trials Overview 245
Nanosyringe – Product Status 245
Nanosyringe – Product Description 245
Nemera La Verpilliere Pipeline Products & Ongoing Clinical Trials Overview 246
Smart Wearable Device – Product Status 246
Smart Wearable Device – Product Description 246
Symbioze – Product Status 247
Symbioze – Product Description 247
Nipro Corp Pipeline Products & Ongoing Clinical Trials Overview 248
Injection Kit – Product Status 248
Injection Kit – Product Description 248
Nordic Group BV Pipeline Products & Ongoing Clinical Trials Overview 249
Nordimet Auto-Injector – Product Status 249
Nordimet Auto-Injector – Product Description 249
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 250
Implantable Ultrasound-Based Blood-Brain-Barrier Device – Product Status 250
Implantable Ultrasound-Based Blood-Brain-Barrier Device – Product Description 250
Nota Laboratories LLC Pipeline Products & Ongoing Clinical Trials Overview 251
Catheter Lock Solution – Product Status 251
Catheter Lock Solution – Product Description 251
NovaXS Biotech Corp. Pipeline Products & Ongoing Clinical Trials Overview 252
Telosis – Product Status 252
Telosis – Product Description 252
Novo Nordisk AS Pipeline Products & Ongoing Clinical Trials Overview 253
Concizumab Pen Injector – Product Status 253
Concizumab Pen Injector – Product Description 254
Novo Nordisk AS – Ongoing Clinical Trials Overview 255
Concizumab Pen Injector – Efficacy and Safety of Concizumab Prophylaxis in Patients with Haemophili
![]()