|1 Table of Contents 3
|1.1 List of Tables 11
|1.2 List of Figures 19
2 Introduction 20
2.1 MRI Systems Overview 20
3 Products under Development 21
3.1 MRI Systems – Pipeline Products by Stage of Development 21
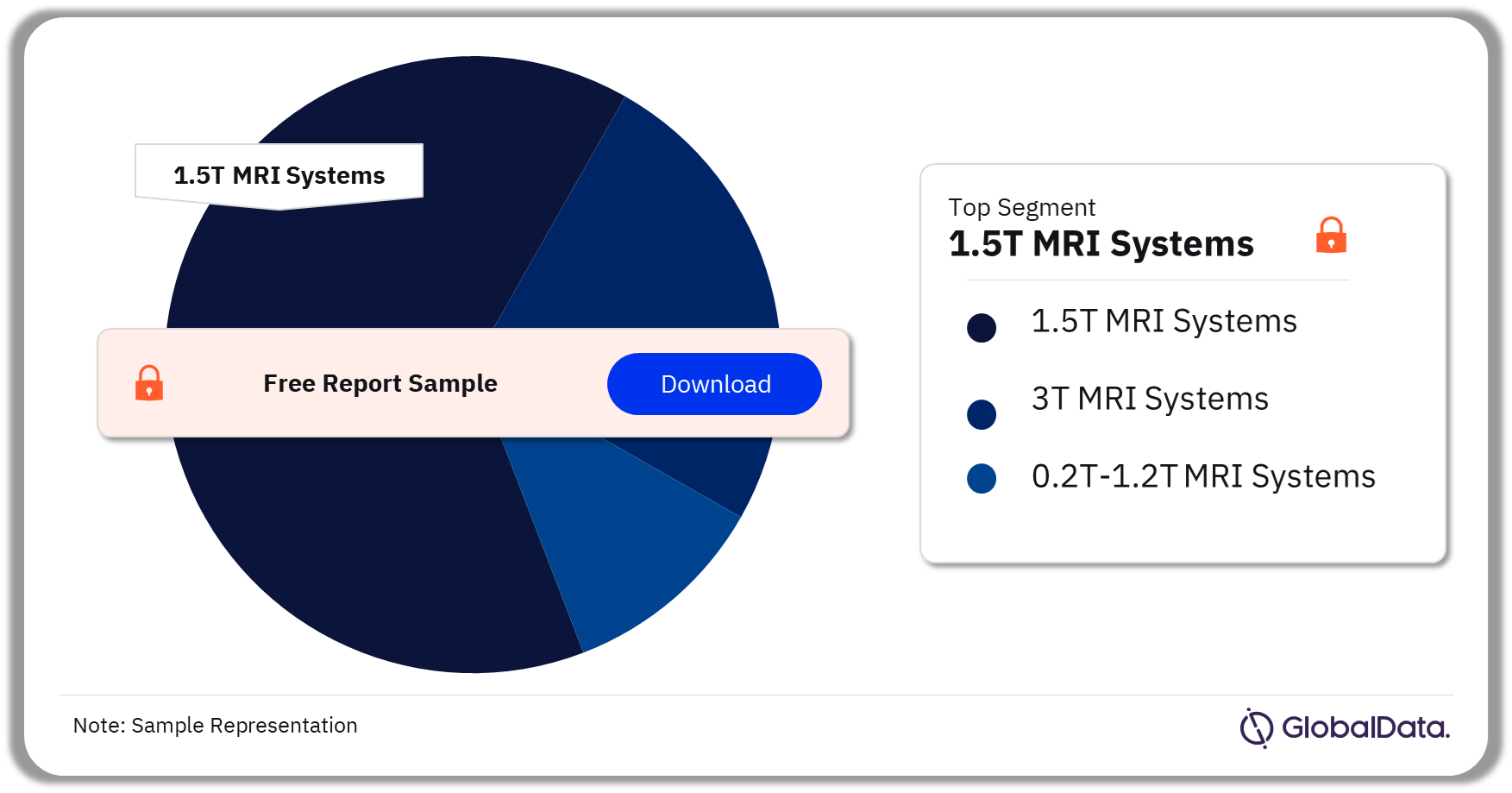
3.2 MRI Systems – Pipeline Products by Segment 22
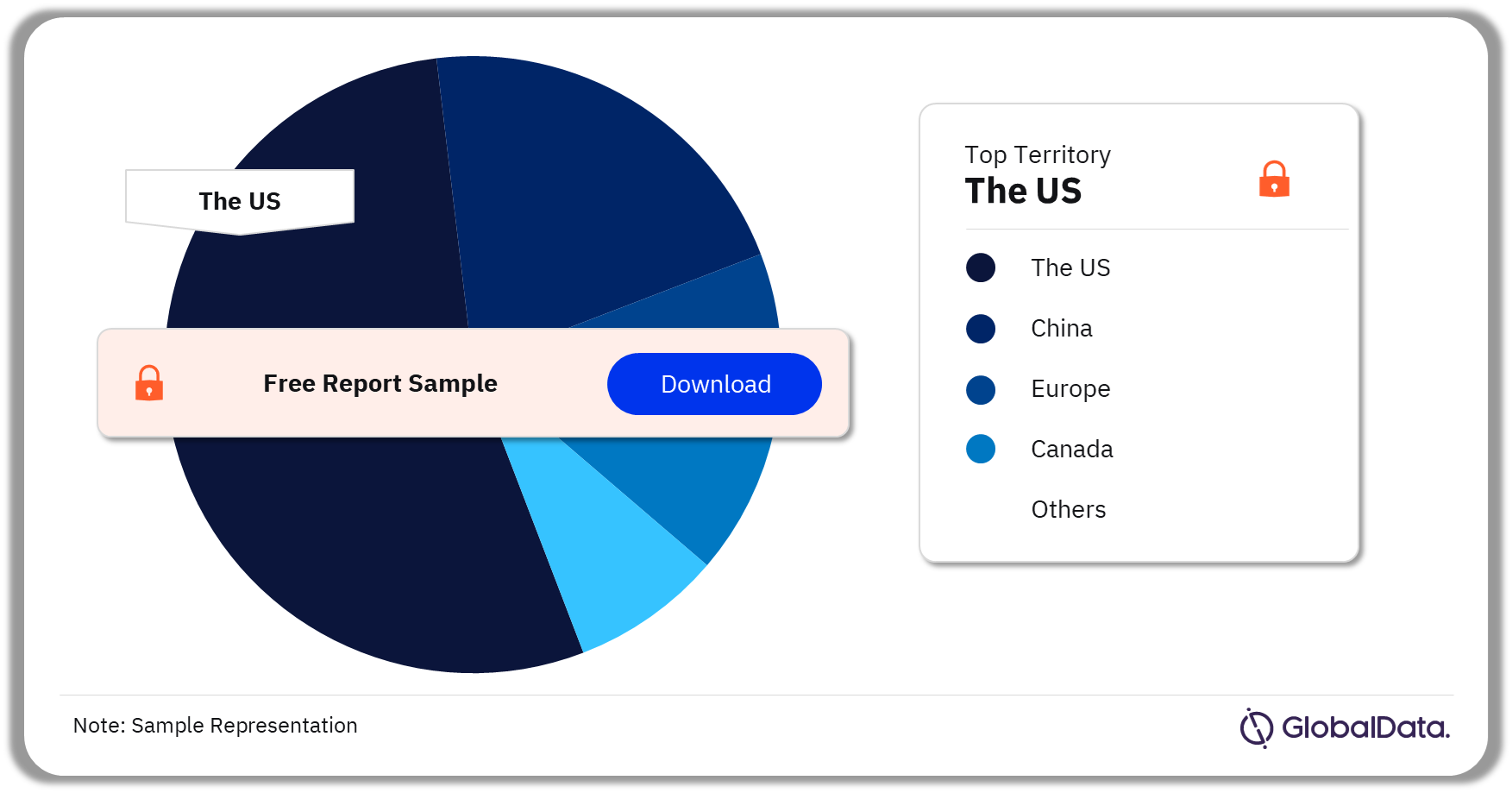
3.3 MRI Systems – Pipeline Products by Territory 23
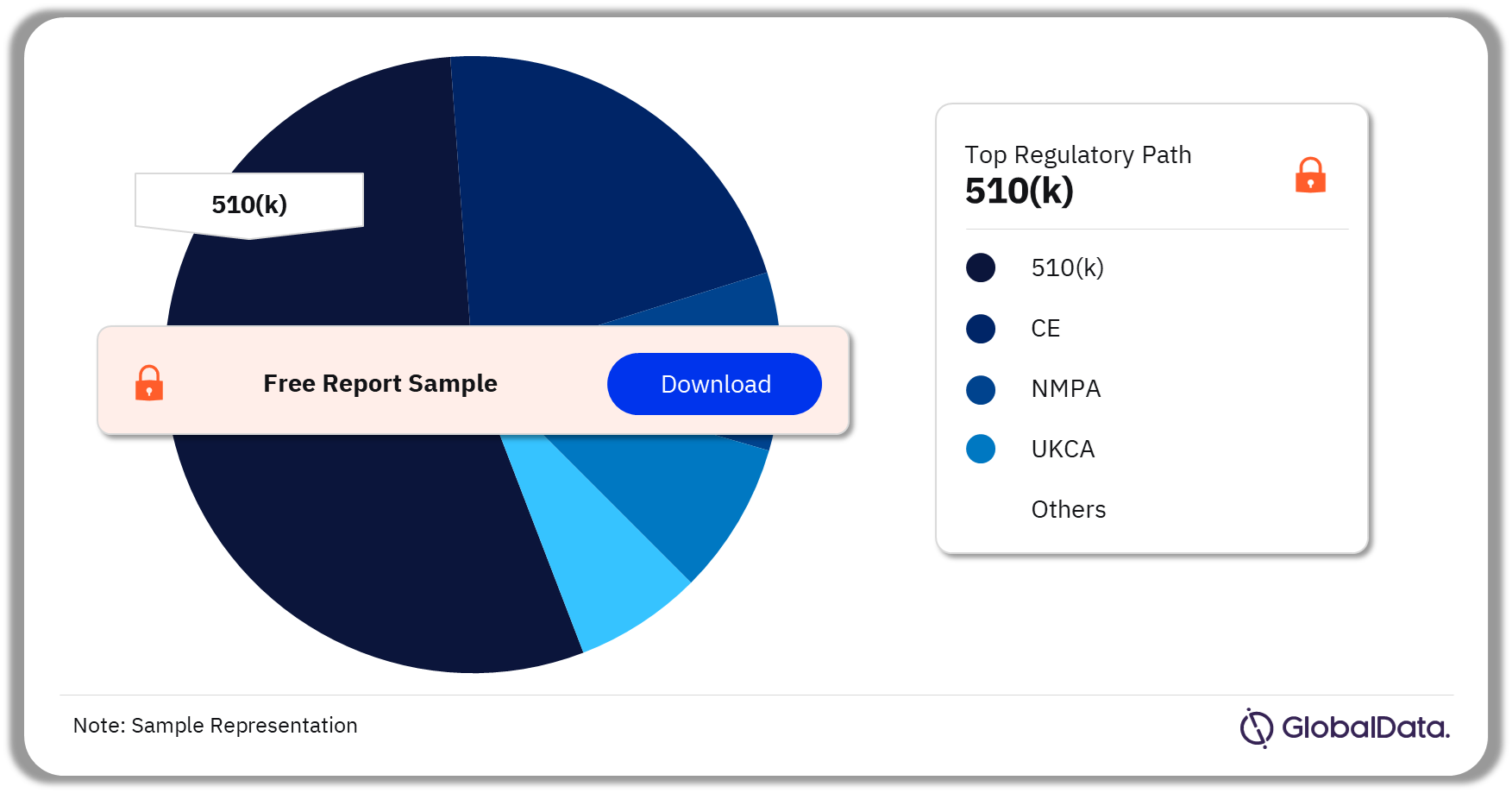
3.4 MRI Systems – Pipeline Products by Regulatory Path 24
3.5 MRI Systems – Pipeline Products by Estimated Approval Date 25
3.6 MRI Systems – Ongoing Clinical Trials 26
4 MRI Systems – Pipeline Products under Development by Companies 27
4.1 MRI Systems Companies – Pipeline Products by Stage of Development 27
4.2 MRI Systems – Pipeline Products by Stage of Development 31
5 MRI Systems Companies and Product Overview 35
5.1 Advanced Biomedical Informatics Group LLC Company Overview 35
5.1.1 Advanced Biomedical Informatics Group LLC Pipeline Products & Ongoing Clinical Trials Overview 35
5.2 Advanced Imaging Research Inc Company Overview 36
5.2.1 Advanced Imaging Research Inc Pipeline Products & Ongoing Clinical Trials Overview 36
5.3 Advanced MRI Technologies Company Overview 37
5.3.1 Advanced MRI Technologies Pipeline Products & Ongoing Clinical Trials Overview 37
5.4 AHS Cancer Control Alberta Company Overview 39
5.4.1 AHS Cancer Control Alberta Pipeline Products & Ongoing Clinical Trials Overview 39
5.5 Anatomyworks LLC Company Overview 40
5.5.1 Anatomyworks LLC Pipeline Products & Ongoing Clinical Trials Overview 40
5.6 ASG Superconductors SpA Company Overview 41
5.6.1 ASG Superconductors SpA Pipeline Products & Ongoing Clinical Trials Overview 41
5.7 Aspect Imaging Inc Company Overview 43
5.7.1 Aspect Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 43
5.8 Boston Children’s Hospital Company Overview 44
5.8.1 Boston Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 44
5.9 Brigham and Women’s Hospital Company Overview 45
5.9.1 Brigham and Women’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 45
5.10 C4 Imaging, LLC Company Overview 48
5.10.1 C4 Imaging, LLC Pipeline Products & Ongoing Clinical Trials Overview 48
5.11 Cincinnati Children’s Hospital Medical Center Company Overview 49
5.11.1 Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 49
5.12 Clear Cut Medical Ltd. Company Overview 50
5.12.1 Clear Cut Medical Ltd. Pipeline Products & Ongoing Clinical Trials Overview 50
5.13 Collagen Medical LLC Company Overview 65
5.13.1 Collagen Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 65
5.14 DeepSpin GmbH Company Overview 66
5.14.1 DeepSpin GmbH Pipeline Products & Ongoing Clinical Trials Overview 66
5.15 Diagnosoft Inc Company Overview 67
5.15.1 Diagnosoft Inc Pipeline Products & Ongoing Clinical Trials Overview 67
5.16 Duke University Company Overview 68
5.16.1 Duke University Pipeline Products & Ongoing Clinical Trials Overview 68
5.17 Eyas Medical Imaging Inc Company Overview 70
5.17.1 Eyas Medical Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 70
5.18 Focal Healthcare Inc Company Overview 71
5.18.1 Focal Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 71
5.19 GE Global Research Company Overview 72
5.19.1 GE Global Research Pipeline Products & Ongoing Clinical Trials Overview 72
5.20 GE HealthCare Technologies Inc Company Overview 74
5.20.1 GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 74
5.21 General Electric Global Research Center Company Overview 76
5.21.1 General Electric Global Research Center Pipeline Products & Ongoing Clinical Trials Overview 76
5.22 Georgetown University Company Overview 77
5.22.1 Georgetown University Pipeline Products & Ongoing Clinical Trials Overview 77
5.23 Harvard University Company Overview 78
5.23.1 Harvard University Pipeline Products & Ongoing Clinical Trials Overview 78
5.24 Hebrew University of Jerusalem Company Overview 79
5.24.1 Hebrew University of Jerusalem Pipeline Products & Ongoing Clinical Trials Overview 79
5.25 Hugo W. Moser Research Institute at Kennedy Krieger Inc Company Overview 80
5.25.1 Hugo W. Moser Research Institute at Kennedy Krieger Inc Pipeline Products & Ongoing Clinical Trials Overview 80
5.26 Hyperfine Inc Company Overview 82
5.26.1 Hyperfine Inc Pipeline Products & Ongoing Clinical Trials Overview 82
5.27 Icahn School of Medicine at Mount Sinai Company Overview 88
5.27.1 Icahn School of Medicine at Mount Sinai Pipeline Products & Ongoing Clinical Trials Overview 88
5.28 Intellidesign Pty Ltd Company Overview 89
5.28.1 Intellidesign Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 89
5.29 Johns Hopkins University Company Overview 90
5.29.1 Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 90
5.30 Koninklijke Philips NV Company Overview 92
5.30.1 Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview 92
5.31 Lausanne University Hospital Company Overview 95
5.31.1 Lausanne University Hospital Pipeline Products & Ongoing Clinical Trials Overview 95
5.32 Leiden University Company Overview 96
5.32.1 Leiden University Pipeline Products & Ongoing Clinical Trials Overview 96
5.33 Leiden University Medical Center Company Overview 97
5.33.1 Leiden University Medical Center Pipeline Products & Ongoing Clinical Trials Overview 97
5.34 Lucidity LLC Company Overview 98
5.34.1 Lucidity LLC Pipeline Products & Ongoing Clinical Trials Overview 98
5.35 Marvel Medtech LLC Company Overview 99
5.35.1 Marvel Medtech LLC Pipeline Products & Ongoing Clinical Trials Overview 99
5.36 Massachusetts General Hospital Company Overview 102
5.36.1 Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 102
5.37 Max Delbruck Center for Molecular Medicine Company Overview 105
5.37.1 Max Delbruck Center for Molecular Medicine Pipeline Products & Ongoing Clinical Trials Overview 105
5.38 Medical College of Wisconsin Company Overview 106
5.38.1 Medical College of Wisconsin Pipeline Products & Ongoing Clinical Trials Overview 106
5.39 MicroMRI Inc Company Overview 108
5.39.1 MicroMRI Inc Pipeline Products & Ongoing Clinical Trials Overview 108
5.40 MRI Robotics LLC Company Overview 109
5.40.1 MRI Robotics LLC Pipeline Products & Ongoing Clinical Trials Overview 109
5.41 MyBrain Co Ltd Company Overview 110
5.41.1 MyBrain Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 110
5.42 National University of Science and Technology MiSiS Company Overview 111
5.42.1 National University of Science and Technology MiSiS Pipeline Products & Ongoing Clinical Trials Overview 111
5.43 Neoscan Solutions GmbH Company Overview 112
5.43.1 Neoscan Solutions GmbH Pipeline Products & Ongoing Clinical Trials Overview 112
5.44 Neuro42 Inc Company Overview 113
5.44.1 Neuro42 Inc Pipeline Products & Ongoing Clinical Trials Overview 113
5.45 Neusoft Medical Systems Co Ltd Company Overview 114
5.45.1 Neusoft Medical Systems Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 114
5.46 New York University School of Medicine Company Overview 115
5.46.1 New York University School of Medicine Pipeline Products & Ongoing Clinical Trials Overview 115
5.47 Newcastle University Company Overview 118
5.47.1 Newcastle University Pipeline Products & Ongoing Clinical Trials Overview 118
5.48 Ningbo Xingaoyi Medical Instruments Co. Ltd Company Overview 119
5.48.1 Ningbo Xingaoyi Medical Instruments Co. Ltd Pipeline Products & Ongoing Clinical Trials Overview 119
5.49 Northwestern University Company Overview 120
5.49.1 Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 120
5.50 Oregon Health & Science University Company Overview 122
5.50.1 Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 122
5.51 Philips Healthcare Company Overview 123
5.51.1 Philips Healthcare Pipeline Products & Ongoing Clinical Trials Overview 123
5.52 PhysioMRI Tech SL Company Overview 124
5.52.1 PhysioMRI Tech SL Pipeline Products & Ongoing Clinical Trials Overview 124
5.53 Prism Clinical Imaging, Inc. Company Overview 125
5.53.1 Prism Clinical Imaging, Inc. Pipeline Products & Ongoing Clinical Trials Overview 125
5.54 Promaxo Inc Company Overview 126
5.54.1 Promaxo Inc Pipeline Products & Ongoing Clinical Trials Overview 126
5.55 Resonance Health Ltd Company Overview 127
5.55.1 Resonance Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 127
5.56 Robin Medical, Inc. Company Overview 130
5.56.1 Robin Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 130
5.57 Shanghai United Imaging Healthcare Co Ltd Company Overview 132
5.57.1 Shanghai United Imaging Healthcare Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 132
5.58 Shanghai United Imaging Intelligence Co Ltd Company Overview 136
5.58.1 Shanghai United Imaging Intelligence Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 136
5.59 Siemens Healthineers AG Company Overview 137
5.59.1 Siemens Healthineers AG Pipeline Products & Ongoing Clinical Trials Overview 137
5.60 Singapore General Hospital Company Overview 145
5.60.1 Singapore General Hospital Pipeline Products & Ongoing Clinical Trials Overview 145
5.61 Stanford University Company Overview 146
5.61.1 Stanford University Pipeline Products & Ongoing Clinical Trials Overview 146
5.62 Superconducting Systems Inc Company Overview 149
5.62.1 Superconducting Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 149
5.63 Synaptive Medical Inc Company Overview 150
5.63.1 Synaptive Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 150
5.64 Tel Aviv University Company Overview 151
5.64.1 Tel Aviv University Pipeline Products & Ongoing Clinical Trials Overview 151
5.65 The University of Manchester Company Overview 152
5.65.1 The University of Manchester Pipeline Products & Ongoing Clinical Trials Overview 152
5.66 The University of Nottingham Company Overview 153
5.66.1 The University of Nottingham Pipeline Products & Ongoing Clinical Trials Overview 153
5.67 Time Medical, Inc. Company Overview 154
5.67.1 Time Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 154
5.68 Tornado Spectral Systems Company Overview 155
5.68.1 Tornado Spectral Systems Pipeline Products & Ongoing Clinical Trials Overview 155
5.69 University of Arizona Company Overview 156
5.69.1 University of Arizona Pipeline Products & Ongoing Clinical Trials Overview 156
5.70 University of California Davis Company Overview 157
5.70.1 University of California Davis Pipeline Products & Ongoing Clinical Trials Overview 157
5.71 University of California Los Angeles Company Overview 158
5.71.1 University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 158
5.72 University of Colorado Company Overview 160
5.72.1 University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 160
5.73 University of Colorado Boulder Company Overview 162
5.73.1 University of Colorado Boulder Pipeline Products & Ongoing Clinical Trials Overview 162
5.74 University of Florida Company Overview 163
5.74.1 University of Florida Pipeline Products & Ongoing Clinical Trials Overview 163
5.75 University of Hong Kong Company Overview 164
5.75.1 University of Hong Kong Pipeline Products & Ongoing Clinical Trials Overview 164
5.76 University of Illinois Company Overview 165
5.76.1 University of Illinois Pipeline Products & Ongoing Clinical Trials Overview 165
5.77 University of Louisville Company Overview 166
5.77.1 University of Louisville Pipeline Products & Ongoing Clinical Trials Overview 166
5.78 University of Michigan Company Overview 167
5.78.1 University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 167
5.79 University of Queensland Company Overview 168
5.79.1 University of Queensland Pipeline Products & Ongoing Clinical Trials Overview 168
5.80 University of Saskatchewan Company Overview 169
5.80.1 University of Saskatchewan Pipeline Products & Ongoing Clinical Trials Overview 169
5.81 University of Strasbourg Company Overview 170
5.81.1 University of Strasbourg Pipeline Products & Ongoing Clinical Trials Overview 170
5.82 University of Texas Southwestern Medical Center Company Overview 171
5.82.1 University of Texas Southwestern Medical Center Pipeline Products & Ongoing Clinical Trials Overview 171
5.83 University of Virginia Company Overview 172
5.83.1 University of Virginia Pipeline Products & Ongoing Clinical Trials Overview 172
5.84 University of Washington Company Overview 173
5.84.1 University of Washington Pipeline Products & Ongoing Clinical Trials Overview 173
5.85 Vanderbilt University Company Overview 175
5.85.1 Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 175
5.86 Varian Medical Systems Inc Company Overview 178
5.86.1 Varian Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 178
5.87 VisionQuest Biomedical LLC Company Overview 179
5.87.1 VisionQuest Biomedical LLC Pipeline Products & Ongoing Clinical Trials Overview 179
5.88 Weinberg Medical Physics LLC Company Overview 180
5.88.1 Weinberg Medical Physics LLC Pipeline Products & Ongoing Clinical Trials Overview 180
5.89 Weizmann Institute of Science Company Overview 181
5.89.1 Weizmann Institute of Science Pipeline Products & Ongoing Clinical Trials Overview 181
5.90 Wipro GE Healthcare Pvt Ltd Company Overview 182
5.90.1 Wipro GE Healthcare Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
5.91 Wright State University Company Overview 184
5.91.1 Wright State University Pipeline Products & Ongoing Clinical Trials Overview 184
6 MRI Systems- Recent Developments 185
6.1 Nov 27, 2023: Siemens Appoints Hakan Ozdemir as CEO of Smart Infrastructure in the Middle East 185
6.2 Nov 26, 2023: Philips Showcases World’s First Mobile MRI System With Helium-Free Operations at RSNA23 185
6.3 Nov 23, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For MAGNETOM Avanto Fit 185
6.4 Nov 23, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For MAGNETOM Skyra Fit 186
6.5 Nov 22, 2023: Oxford Instruments Brings Hill & Smith CFO Hannah Nichols to Board 186
6.6 Nov 22, 2023: Oxford Instruments Names Hill & Smith CFO to Its Board 186
6.7 Nov 22, 2023: Oxford Instruments Announces Appointment of Independent Non-Executive Director 186
6.8 Nov 20, 2023: Hyperfine Announces Presentations on Clinical Advancements at RSNA 2023 186
6.9 Nov 20, 2023: Concord Medical Schedules 2023 Annual Meeting of Shareholders 187
6.10 Nov 17, 2023: SyntheticMR: Nomination Committee Appointed for the 2024 Annual General Meeting 187
6.11 Nov 14, 2023: Fonar Announces Financial Results for the First Quarter of Fiscal 2024 188
6.12 Nov 14, 2023: Oxford Instruments Reports Interim Results for the Six Months to 30 September 2023 190
6.13 Nov 10, 2023: SyntheticMR Announces Interim Report July – September 2023 191
6.14 Nov 08, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For Magnetom Lumina System 192
6.15 Nov 08, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For MAGNETOM Aera 192
6.16 Nov 08, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For MAGNETOM Skyra 192
6.17 Nov 08, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For Magnetom Prisma MRI Scanner 193
6.18 Nov 08, 2023: Siemens Healthineers Receives Additional 510(K) Clearance For MAGNETOM Prisma-Fit 193
6.19 Nov 06, 2023: Siemens Healthineers Receives Additional 510(K) Clearance for MAGNETOM Vida 193
6.20 Nov 06, 2023: Butterfly Network to Present at the Jefferies London Healthcare Conference 194
6.21 Nov 02, 2023: Butterfly Network Reports Third Quarter 2023 Financial Results 194
6.22 Oct 31, 2023: GE HealthCare Reports Third Quarter 2023 Financial Results 195
6.23 Oct 26, 2023: Hyperfine Receives Additional 510(K) Clearance For Swoop Portable MR Imaging System 197
6.24 Oct 26, 2023: ContextVision Reports Q3 Results 197
6.25 Oct 25, 2023: Esaote’s New S-Scan Open Redefines Excellence in Magnetic Resonance Imaging 198
6.26 Oct 19, 2023: Ascelia Pharma Notice of Extraordinary General Meeting 198
6.27 Oct 18, 2023: Butterfly Network to Report Third Quarter 2023 Financial Results on November 2, 2023 204
6.28 Oct 13, 2023: Smil Southwest Medical Imaging Announces the First GE Healthcare Signa Hero MRI Scanner in Arizona 204
6.29 Oct 09, 2023: Hyperfine Receives FDA Clearance for Updated AI-powered Software with Improved Image Quality for All Swoop System Sequences 205
6.30 Oct 09, 2023: Thinksono Joins Butterfly Network’s AI Marketplace 206
6.31 Oct 03, 2023: One Call Names Howard Cutler Chief Strategy & Network Officer 206
6.32 Oct 02, 2023: Promaxo Announces Sale of 20 Systems in the Third Quarter 2023 207
6.33 Oct 02, 2023: Oxford Instruments Welcomes New Chief Executive, Richard Tyson 207
6.34 Sep 28, 2023: FONAR Announces Financial Results for Fiscal 2023 208
6.35 Sep 26, 2023: Promaxo Receives 510(k) Clearance for Promaxo MRI System II 211
6.36 Sep 22, 2023: Concord Medical Reports Financial Results for the First Half of 2023 211
6.37 Sep 21, 2023: Siemens Healthineers Receives 510(k) Clearance for Magnetom Viato.Mobile 213
6.38 Sep 20, 2023: Aspect Imaging Receives Innovative Technology Contract from Vizient for Embrace Point of Care Neonatal MRI System 213
6.39 Sep 18, 2023: Hitachi Vantara Appoints New GM for Sub-Saharan Africa 214
6.40 Sep 14, 2023: Vista.ai Names Steve Cashman to Board of Directors 214
6.41 Sep 12, 2023: Hitachi Makes Executive Responsibility Change 214
6.42 Sep 08, 2023: Polarean Imaging : Partnership with VIDA To Streamline Adoption of Advanced MRI of the Lungs 214
6.43 Sep 07, 2023: Philips Reaches Resolution of US Economic Loss Litigation 215
6.44 Sep 06, 2023: Elucid Appoints Windi Hary to Lead Regulatory Affairs and Quality Management 216
6.45 Aug 29, 2023: CMS grants reimbursement code for the Polarean XENOVIEW™ MRI Technology 216
6.46 Aug 28, 2023: ContextVision Appoints Richard Hallstrom as Chief Financial Officer 217
6.47 Aug 25, 2023: Asiamedic Sets New Standard in Healthcare With Installation of First Signa Hero
3.0T MRI Scanner in Asia-Pacific 217
6.48 Aug 24, 2023: ContextVision Announces Strong Second Quarter With Solid Margin Growth 218
6.49 Aug 22, 2023: SyntheticMR Announces Interim Report April – June 2023 219
6.50 Aug 16, 2023: Philips Medical Systems Receives an Additional 510(k) Clearance for Ingenia Elition 219
6.51 Aug 10, 2023: Prenuvo AI-Assisted Study Uncovers Novel Correlation Between Lifestyle and Brain Volume Changes 220
6.52 Aug 10, 2023: Promaxo Appoints Chad Zaring, Chief Commercial Officer, and Matt Collins, Vice President of Marketing 221
6.53 Aug 09, 2023: Enrollment Completed for HOPE PMR Pediatric Hydrocephalus Study Using Hyperfine, Portable MR Imaging System 221
6.54 Aug 07, 2023: Sarvodaya Hospital in India deploys Fujifilm’s smart MRI machine 222
6.55 Aug 07, 2023: Game-changing Indian MRI Scanner Launched: Targets 6 billion People without Access to Cutting-edge Imaging Technologies 222
6.56 Jul 28, 2023: CoxHealth and Philips partner on virtual care solution 223
6.57 Jul 24, 2023: Fujifilm Healthcare Receives FDA 510(K) Clearance for ECHELON Synergy MRI System 224
6.58 Jul 17, 2023: ViewRay Files Voluntary Chapter 11 Petitions 224
6.59 Jul 15, 2023: Butterfly Network Announces Lay Off 225
6.60 Jul 13, 2023: Eyas installs neonatal MRI system at Cincinnati Children’s NICU 225
6.61 Jul 12, 2023: Hyperfine begins Swoop system’s multi-site observational trial 226
6.62 Jul 10, 2023: Springbok Analytics Announces Advanced Mobile MRI Capabilities for Elite Sports Organizations 226
6.63 Jul 01, 2023: Siemens Healthcare names Hariharan Subramanian as the Managing Director 227
6.64 Jun 23, 2023: GE HealthCare Advances PET/MR Capabilities with AIR Technologies 227
6.65 Jun 23, 2023: GE HealthCare Advances PET/MR Capabilities with the AIR Technologies 228
6.66 Jun 13, 2023: Shanghai United Imaging Healthcare Receives Additional 510(k) Clearance for uMR Omega 229
6.67 Jun 13, 2023: Oxford Instruments Announcement of unaudited full-year results for the 12 months to 31 March 2023 229
6.68 Jun 13, 2023: Oxford Instruments Announces Preliminary Results for the 12 Months to 31 March 2023 231
6.69 Jun 06, 2023: icometrix awarded a grant by The Michael J. Fox Foundation to research brain MRI biomarkers for Parkinson’s disease 231
6.70 Jun 04, 2023: Shanghai United Imaging Healthcare receives additional 510(k) clearance for uMR 570 232
6.71 Jun 02, 2023: Philips breakthrough MR 7700 system adds Xenon capabilities to enhance ventilation imaging at ISMRM 2023 232
6.72 Jun 02, 2023: United Imaging to Unveil Ultra-Modern MRI Technology Platform at ISMRM in Toronto 234
6.73 Jun 01, 2023: Hitachi Announces Executive Responsibility Change 234
6.74 May 24, 2023: SyntheticMR Report from Annual General Meeting 234
6.75 May 24, 2023: Siemens Healthineers invests 80 million euros in new semiconductor factory in Forchheim 235
6.76 May 23, 2023: SyntheticMR announces interim report January – March 2023 236
6.77 May 22, 2023: New Imaging Platform for MS Preclinical Trials 237
6.78 May 18, 2023: Hyperfine, receives Grant for an additional three years to utilize innovative Swoop Portable MR Imaging System in low and middle-income countries 238
6.79 May 17, 2023: United Imaging unveils a full portfolio of products at CMEF 2023, Highlighting Its AI Capabilities across Modalities 239
6.80 May 16, 2023: Jefferson Radiology implements groundbreaking MRI technology to reduce exam time by up to half 240
6.81 May 15, 2023: GE HealthCare launches new solutions for radiation therapy 240
6.82 May 15, 2023: iCAD reports financial results for first quarter ended March 31, 2023 241
6.83 May 15, 2023: Fonar announces financial results for fiscal 2023 3rd quarter and nine-month period 243
6.84 May 10, 2023: ViewRay announces first quarter 2023 results 244
6.85 May 09, 2023: ViewRay Announces announces conference call for first quarter 2023 financial results to be held after market on may 10, 2023 246
6.86 May 09, 2023: GE HealthCare Names James Saccaro Vice President and Chief Financial Officer 246
6.87 May 04, 2023: Philips Medical Systems obtains an additional 510(k) clearance the Ingenia Elition 246
6.88 May 04, 2023: Koninklijke Philips obtains an additional 510(k) clearance the MR 7700 System 247
6.89 May 04, 2023: Philips’ BlueSeal helium-free MR operations magnet wins Best New Technology Solution for Radiology award 247
6.90 May 04, 2023: Ascelia Pharma Announces Bulletin from the Annual General Meeting 248
6.91 Apr 28, 2023: Siemens to announce second quarter results FY 2023 250
6.92 Apr 27, 2023: GE HealthCare expands contrast media portfolio with launch of MRI agent Pixxoscan (gadobutrol) 250
6.93 Apr 27, 2023: Hitachi reports financial results for year ended March 31, 2023 251
6.94 Apr 25, 2023: Mediso launches next generation MRI spectrometer spinScan 252
6.95 Apr 25, 2023: Siemens, Siemens Healthineers and UCSF research partnership proves significant energy, cost and emission reduction in MRI machine operation 252
6.96 Apr 22, 2023: Philips Medical Systems obtains additional 510(k) clearance the MR 5300 System 253
6.97 Apr 22, 2023: Philips Medical Systems obtains additional 510(k) clearance the MR 7700 System 253
6.98 Apr 22, 2023: Philips Medical Systems obtains additional 510(k) clearance the Ingenia Elition 254
6.99 Apr 22, 2023: Philips Healthcare obtains additional 510(k) clearance the Ingenia
1.5T CX 254
6.100 Apr 22, 2023: Philips Healthcare obtains additional 510(k) clearance the Ingenia
1.5T 254
6.101 Apr 21, 2023: Philips Medical Systems obtains additional 510(k) clearance for Ingenia Ambition MR System 255
6.102 Apr 20, 2023: Prism Clinical Imaging and Imaging Biometrics announce relationship 255
6.103 Apr 19, 2023: Concord Medical Files 2022 Annual Report on Form 20-F 255
6.104 Apr 17, 2023: iCAD Strengthens Leadership Team with Appointment of Chief Financial Officer and New Senior Executives 256
6.105 Apr 14, 2023: Siemens Healthineers receives additional 510(k) clearance for MAGNETOM Sempra 257
6.106 Apr 14, 2023: Notice to attend the annual general meeting of SyntheticMR AB 257
6.107 Apr 13, 2023: ViewRay Announces Preliminary First Quarter 2023 Results and Updated Fiscal Year 2023 Financial Guidance; Company to Explore Strategic Alternatives to Maximize Shareholder Value 262
6.108 Apr 13, 2023: Ascelia Pharma Publishes Annual Report for 2022 263
6.109 Apr 13, 2023: Ian Barkshire to retire and Richard Tyson to be appointed as Chief Executive Officer of Oxford Instruments 264
6.110 Apr 12, 2023: ViewRay Announces Conference Call for Preliminary First Quarter 2023 Financial Results to be held Pre-Market opening on April 13, 2023 265
6.111 Apr 11, 2023: SyntheticMR publishes annual report for 2022 265
6.112 Apr 06, 2023: One Call Adds Two Veteran Executives to Board of Directors 266
6.113 Apr 04, 2023: Calyx delivers imaging biomarkers to advance neuropsychiatric treatment development 266
6.114 Apr 03, 2023: Notice of the annual general meeting in Ascelia Pharma AB 266
6.115 Mar 31, 2023: RAYUS Radiology adds new wide-Bore 3T MRI Scanner to Its fleet of industry-leading imaging solutions 270
6.116 Mar 28, 2023: Siemens Healthineers receives additional 510(k) clearance for MAGNETOM Amira 270
6.117 Mar 27, 2023: iCad lays off 28% of its workforce in restructuring plan 271
6.118 Mar 17, 2023: Bruker announces two
1.2 GHz NMR Systems orders from the UK 271
6.119 Mar 13, 2023: Hitachi Announces Executive Responsibility Changes 271
6.120 Mar 13, 2023: iCAD Announces Leadership Transition 272
6.121 Mar 13, 2023: Ascelia Pharma Mourns the Passing of Board Member Rene Spogard 272
6.122 Mar 13, 2023: iCAD Previews Fourth Quarter Financial Results, Full Report on March 28, 2023 272
6.123 Mar 10, 2023: Time Medical receives 510(k) clearance for NEONA
1.5T MRI System 273
6.124 Mar 10, 2023: Contract win for a Phase 1/2 rare neurodegenerative disease gene therapy study; value c.£0.7m. 273
6.125 Mar 09, 2023: Siemens Healthineers receives an additional 510(k) clearance for MAGNETOM Vida 273
6.126 Mar 06, 2023: Synaptive Medical awarded inaugural INOVAIT Focus Fund investments to fund MRI Project 274
6.127 Mar 06, 2023: Medic Vision’s AI-assisted technology improves efficiency at North Texas Imaging center 274
6.128 Mar 06, 2023: IMEXHS wins new radiology services contract 275
6.129 Mar 03, 2023: Nation’s first pediatric hybrid intraoperative MRI neurosurgery suite opens at Children’s Minnesota 276
6.130 Mar 02, 2023: United Imaging Healthcare unveils the world’s first whole-body ultra-high field
5.0T MRI, uMR Jupiter
5.0T, at ECR 276
6.131 Mar 02, 2023: Siteman Cancer Center at Washington University Treats 2,000th Patient with MRIdian MRI-Guided Radiation Therapy 277
6.132 Mar 01, 2023: Philips highlights AI-powered integrated diagnostic approach at ECR 2023 278
6.133 Feb 28, 2023: GE Healthcare receives 510(k) clearance for SIGNA Victor MRI Scanner 279
6.134 Feb 28, 2023: Hyperfine receives FDA clearance for Updated Software to further improve Diffusion-Weighted Imaging 279
6.135 Feb 27, 2023: ViewRay Announces Fourth Quarter and Full Year 2022 Results 280
6.136 Feb 21, 2023: Shanghai United Imaging Healthcare receives 510(k) clearance for uMR 680 281
6.137 Feb 21, 2023: Hyperfine, Swoop Portable MR Imaging System receives CE Marking after meeting comprehensive new EU MDR regulations 282
6.138 Feb 16, 2023: Promaxo announces clinical collaboration with Mount Sinai Health System and UTHealth Houston 282
6.139 Feb 14, 2023: Siemens Limited announces Q1 FY 2023 results; 80% increase in PAT from continuing operations 283
6.140 Feb 13, 2023: Hyperfine, announces FDA clearance for improved AI-powered software and expanded field of view for the Swoop Portable MR Imaging System 283
6.141 Feb 09, 2023: Latest data demonstrate potential of the Swoop Portable MR Imaging System in the follow-up of stroke patients who have received a thrombectomy 284
6.142 Feb 08, 2023: Siemens Announces Earnings Release and Financial Results for the First Quarter of Fiscal Year 2023 285
6.143 Feb 08, 2023: Siemens Announces Earnings Release Q1 FY 2023 285
6.144 Feb 06, 2023: Hyperfine Swoop Point-of-Care MRI System takes the stage at ISMRM with twelve abstract inclusions 288
6.145 Feb 03, 2023: AI-Automated MRI planning solution launched to beat waiting-list backlogs & support efficient UK community diagnostic strategies 288
6.146 Feb 02, 2023: NIH-funded researchers develop new MRI technique for pregnant patients 289
6.147 Feb 01, 2023: Hitachi Announces Executive Changes 289
6.148 Feb 01, 2023: Hitachi Annnounces Consolidated Financial Results for the Third Quarter Ended December 31, 2022 290
6.149 Feb 01, 2023: Canon posts record revenues in medical business unit 290
6.150 Jan 30, 2023: Philips to lay off 6,000 employees in drive to improve profitability 290
6.151 Jan 30, 2023: GE HealthCare Reports Fourth Quarter and Full Year 2022 Financial Results 290
6.152 Jan 26, 2023: Elucid Appoints Scott Burger as Chief Commercial Officer 293
6.153 Jan 26, 2023: CU research team moves one step closer to printing models of life-like 3D organs 293
6.154 Jan 24, 2023: Portable cap can measure cognition with pulsed laser light 294
6.155 Jan 17, 2023: GE HealthCare to Announce Fourth Quarter and Full Year 2022 Results on January 30, 2023 296
6.156 Jan 10, 2023: GE HealthCare Announces Preliminary Fourth Quarter and Full Year 2022 Revenue Results; Introduces 2023 Outlook 296
6.157 Jan 09, 2023: ViewRay Announces Preliminary Fourth Quarter and Full Year 2022 Results; appoints William P. “Bill” Burke as Chief Financial Officer 297
6.158 Jan 08, 2023: Hologic Announces Preliminary Revenue Results for First Quarter of Fiscal 2023 298
6.159 Jan 02, 2023: Koninklijke Philips receives additional 510(k) clearance for MR 7700 300
6.160 Jan 02, 2023: Philips Medical Systems obtains additional 510(k) clearance for MR 5300 System 300
6.161 Dec 21, 2022: Resonance Health lodges MRI fibrosis provisional patent application 300
6.162 Dec 20, 2022: Hyperfine Research receives an additional 510(k) clearance for the Hyperfine Swoop Portable MRI System 302
6.163 Dec 20, 2022: Promaxo Announces Appointment of Dr. Prithipal S. Sethi as Chief Medical Officer of Urology 302
6.164 Dec 09, 2022: IXICO : Contract win for an early phase Multiple System Atrophy disease trial 302
6.165 Dec 07, 2022: New MRI scanner aims to deliver workflow and productivity fortitude to UK healthcare 303
6.166 Dec 07, 2022: Hitachi to hire 30,000 tech workers, defying global wave of layoffs 303
6.167 Dec 06, 2022: US Radiology Specialists enhances imaging capabilities at its American Health Imaging Centers in Alabama & Georgia with the addition of new MROpen EVO MRI systems from ASG Superconductors 303
6.168 Dec 06, 2022: Butterfly Announces CEO Transition 304
7 Appendix 305
7.1 Methodology 305
7.2 About GlobalData 308
7.3 Contact Us 308
7.4 Disclaimer 308
![]()