Dry Powder Inhaler Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Dry Powder Inhaler Devices Pipeline Market Report Overview
Dry powder inhaler devices deliver medication to the lungs in the form of a dry powder. The Dry Powder Inhaler Devices pipeline market research report provides comprehensive information about the Dry Powder Inhaler Devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Territories | · The US
· China · Europe · Global · Australia |
| Key Regulatory Paths | · 510(k)
· NMPA · MDL · CE · TGA |
| Leading Companies | · Aespira Ltd.
· AKELA Pharma Inc. (Inactive) · Bayer AG · Cambridge Healthcare Innovations Ltd · Creare LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Dry Powder Inhaler Devices Pipeline Market Segmentation by Territories
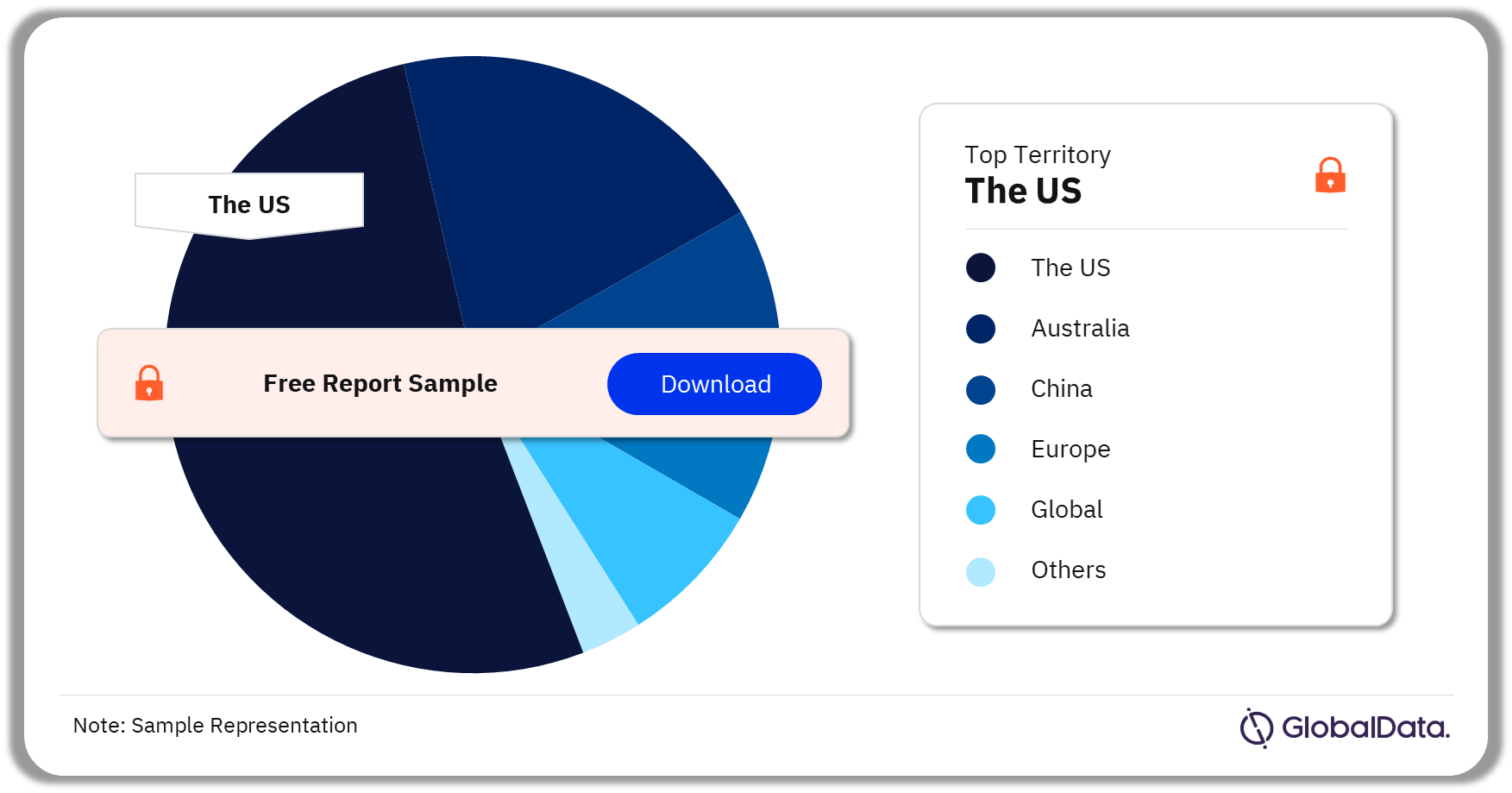
Some of the key territories in the Dry Powder Inhaler Devices market are China, the United States, Europe, Global, and Australia among others. As of November 2023, the US has the highest number of pipeline products.
Dry Powder Inhaler Devices Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Dry Powder Inhaler Devices Pipeline Market
Dry Powder Inhaler Devices Pipeline Market Segmentation by Regulatory Paths
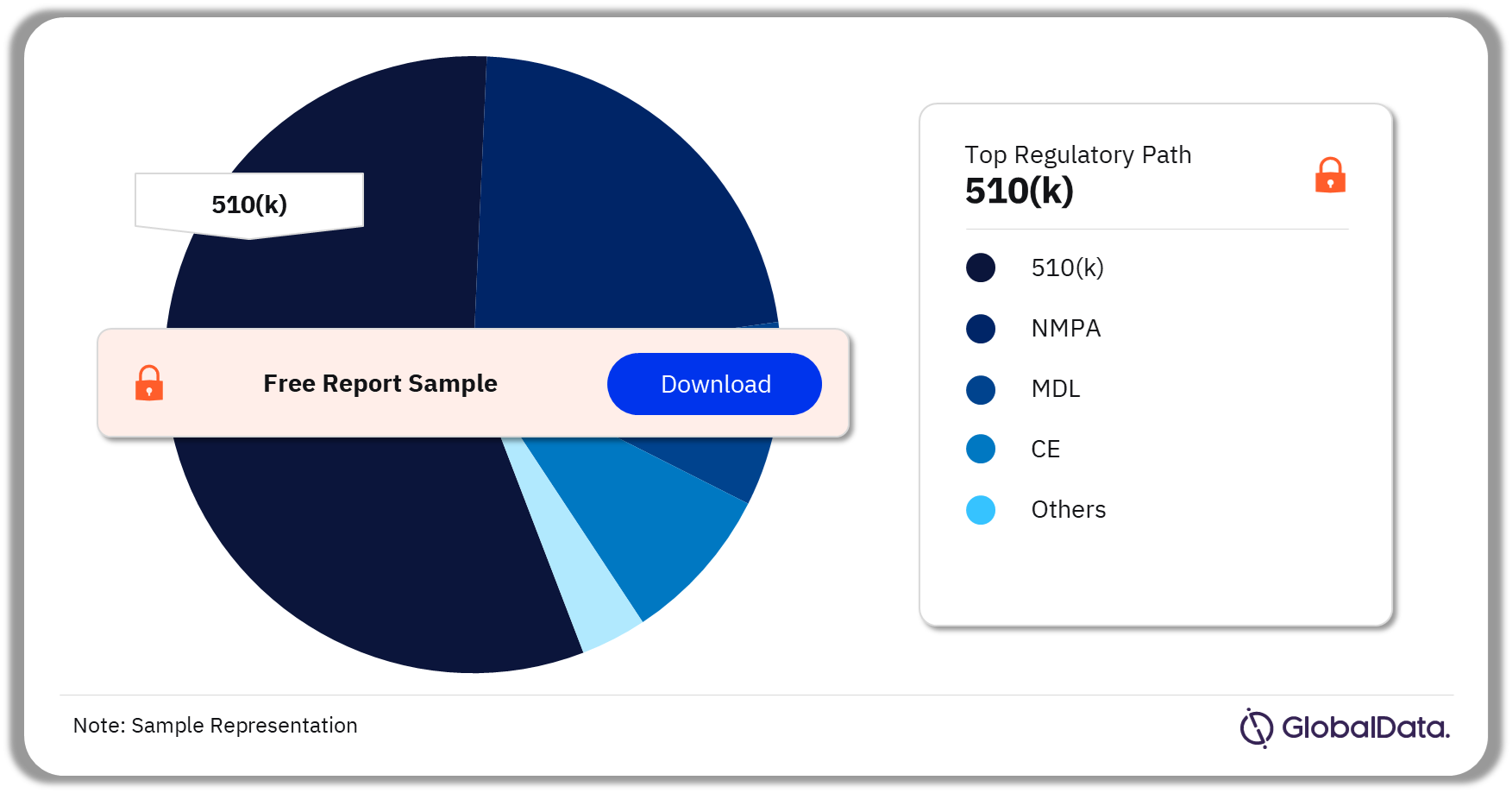
Some of the key regulatory paths in the Dry Powder Inhaler Devices pipeline market are 510(k), NMPA, MDL, CE, and TGA among others. As of November 2023, 510(k) is the most followed pathway for pipeline products in the market.
Dry Powder Inhaler Devices Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Dry Powder Inhaler Devices Pipeline Market
Dry Powder Inhaler Devices Pipeline Market – Competitive Landscape
Some of the key companies in the Dry Powder Inhaler Devices pipeline market are:
- Aespira Ltd.
- AKELA Pharma Inc. (Inactive)
- Bayer AG
- Cambridge Healthcare Innovations Ltd
- Creare LLC
Bayer AG (Bayer): It is engaged in the discovery, development, manufacturing, and commercialization of products for human health, and agriculture. It provides medicines for cardiovascular diseases, women’s health, cancer, hematology, ophthalmology, and other indications. It also strives to develop new molecules and technologies for use in the fields of medicine and modern agriculture. The company’s product portfolio includes prescription products, specialty pharmaceuticals, diagnostic imaging equipment, non-prescription (over-the-counter or OTC) products, seeds, crop protection solutions, and non-agricultural pest control solutions. Bayer markets its healthcare and crop protection products essentially through wholesalers, pharmacies, hospitals, and retailers. It operates through a network of subsidiaries in Asia-Pacific, Europe, North America, Latin America, Africa, and the Middle East. Bayer is headquartered in Leverkusen, North Rhine-Westphalia, Germany.
Dry Powder Inhaler Devices Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Dry Powder Inhaler Devices Pipeline Market
Segments Covered in the Report
Dry Powder Inhaler Devices Pipeline Market Territories Outlook
- The US
- China
- Europe
- Global
- Australia
Dry Powder Inhaler Devices Pipeline Market Regulatory Paths Outlook
- 510(k)
- NMPA
- MDL
- CE
- TGA
Scope
This report provides:
- Extensive coverage of the Dry Powder Inhaler Devices under development.
- Review details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in pipeline product development and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data related to ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
This report provides:
- Extensive coverage of the Dry Powder Inhaler Devices under development.
- Review details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in pipeline product development and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data related to ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Advent Pharmaceuticals Pty Ltd
Aespira Ltd.
AKELA Pharma Inc. (Inactive)
Bayer AG
Cambridge Healthcare Innovations Ltd
Creare LLC
DMK Pharmaceuticals Corp
Eli Lilly and Co
Glenmark Pharmaceuticals Ltd
GSK plc
Hovione Technology Ltd
Iconovo AB
Lupin Pharmaceuticals Inc
MannKind Corp
Monash University
Nektar Therapeutics
Ology Bioservices Inc
OPKO Health Inc
OtiTopic Inc
Phargentis SA
Pharmaxis Ltd
PureIMS BV
Quench Medical Inc
Respira Therapeutics Inc
Respirent Pharmaceuticals Co Ltd
Sandoz International GmbH
Sheffield Hallam University
Shin Nippon Biomedical Laboratories Ltd
Spyryx Biosciences Inc (Inactive)
Teva Pharmaceutical Industries Ltd
Transpire Bio Inc
University of Kansas
University of Sydney
University of Texas Medical Branch at Galveston
Vectura Group Plc
Verona Pharma Plc
Virginia Commonwealth University
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory has the highest number of pipeline products in the Dry Powder Inhaler Devices pipeline market?
As of November 2023, the US has the highest number of products in the Dry Powder Inhaler Devices pipeline market.
-
Which is the most followed regulatory pathway in the Dry Powder Inhaler Devices pipeline market?
As of November 2023, 510(k) is the most followed pathway for pipeline products in the Dry Powder Inhaler Devices market.
-
Who are the major companies operating in the Dry Powder Inhaler Devices pipeline market?
Some of the key companies in the Dry Powder Inhaler Devices pipeline market are Aespira Ltd., AKELA Pharma Inc. (Inactive), Bayer AG, Cambridge Healthcare Innovations Ltd, and Creare LLC among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Dry Powder Inhaler Devices reports