Computed Tomography (CT) Systems Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Computed Tomography Systems Pipeline Market Report Overview
Computed Tomography (CT) is a powerful, non-destructive evaluation technique used for producing 2D and 3D cross-sectional images of an object from a flat X-ray image. It is used on patients with a high risk of colon cancer, or full-motion heart scans for patients with a high risk of heart disease. It can also be used to image complex fractures, detect infarction, tumors, calcifications, hemorrhage, bone trauma, and also acute and chronic changes in the lung parenchyma, etc. The device includes a slice detector and an X-ray source. 256-320 slice CT Systems, 128-slice CT Systems, 64-slice CT Systems, 20-40 slice CT Systems, and 16-slice and below CT Systems are tracked under this category. Tracked here under is the equipment only and not accessories and/or additional costs associated with CT installation.
The computed tomography systems pipeline market research report provides comprehensive information about the computed tomography systems pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · 64-slice CT Systems
· 16-slice and below CT Systems |
| Key Territories | · The US
· Europe · China · Japan · Australia |
| Key Regulatory Paths | · 510(k)
· NMPA · CE · UKCA · TGA |
| Leading Companies | · Advanced Breast-CT GmbH
· Annalise-AI Pty Ltd · Bioptics Technology LLC · California Institute of Technology · Canon Medical Systems Corp · Canon Medical Systems USA Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Computed Tomography Systems Pipeline Market Segments
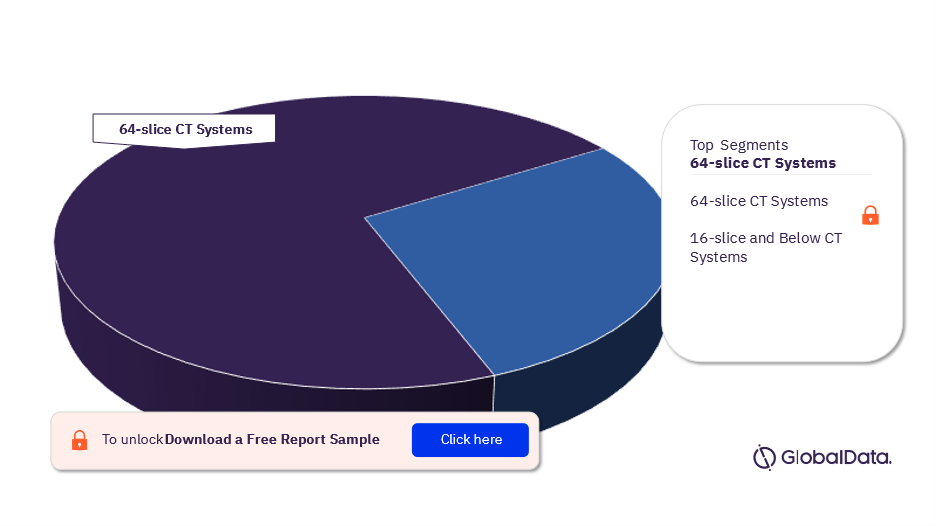
Some of the key segments in the computed tomography systems pipeline market are 64-slice CT systems and 16-slice and below CT systems. As of May 2023, 64-slice CT systems have the highest number of pipeline products.
Computed Tomography Systems Pipeline Market Analysis, by Segments, 2023 (%)
For more segment insights into the computed tomography systems pipeline market, download a free report sample
Computed Tomography Systems Pipeline Market Segmentation by Territories
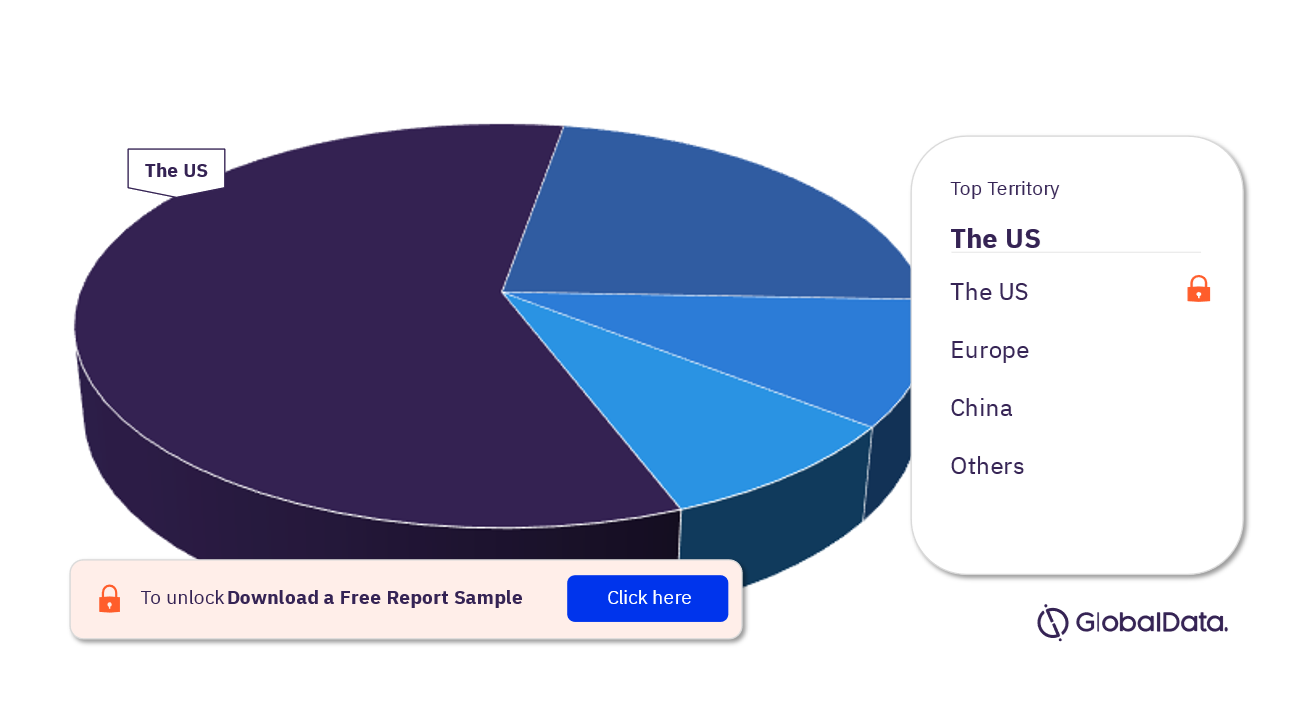
Some of the territories in the computed tomography systems pipeline market are the US, Europe, China, Japan, and Australia among others. As of May 2023, the US has the highest number of pipeline products.
Computed Tomography Systems Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the computed tomography systems pipeline market, download a free report sample
Computed Tomography Systems Pipeline Market Segmentation by Regulatory Paths
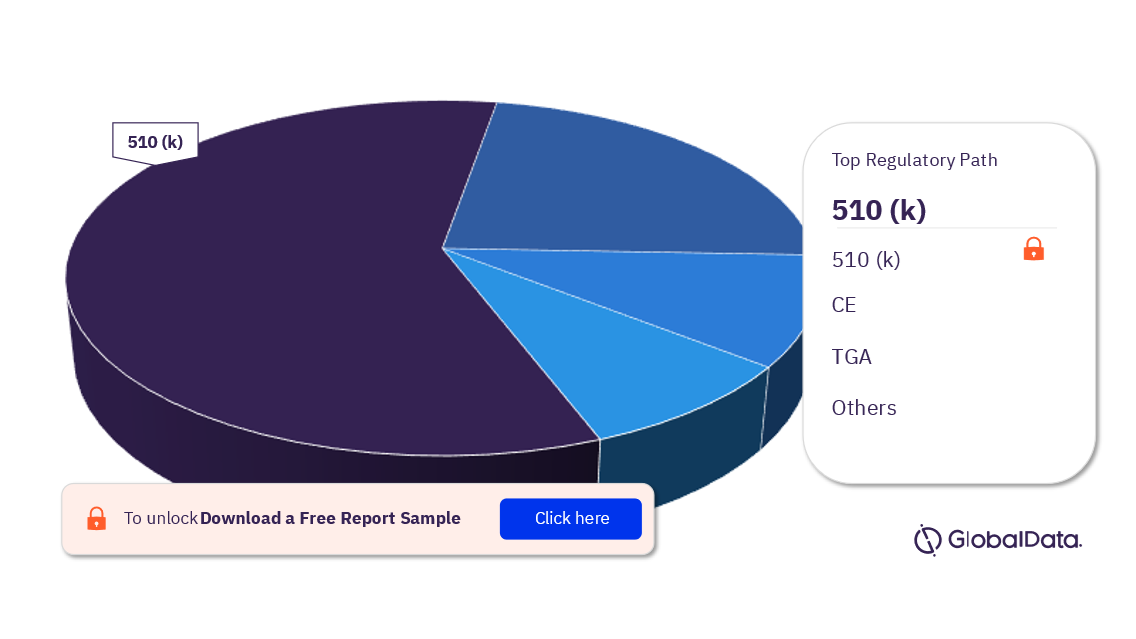
Some of the regulatory paths in the computed tomography systems pipeline market are 510(k), NMPA, UKCA, TGA, and CE. As of May 2023, 510(k) is the most followed pathway for pipeline products.
Computed Tomography Systems Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory path insights into the computed tomography systems pipeline market, download a free report sample
Computed Tomography Systems Pipeline Market – Competitive Landscape
Some of the key companies in the computed tomography systems pipeline market are Advanced Breast-CT GmbH, Annalise-AI Pty Ltd, Bioptics Technology LLC, California Institute of Technology, Canon Medical Systems Corp, and Canon Medical Systems USA Inc among others.
Annalise-AI Pty Ltd Company Overview: Annalise-AI Pty Ltd (Annalise) is a developer of AI products to improve the delivery of medical imaging services. Annalise is headquartered in Sydney, New South Wales, Australia. Annalise CT Chest is intended for computed tomography image analysis. It is designed to identify and localize more than 100 clinical findings on CT chest images. It is based on Artificial Intelligence Technology.
Segments Covered in the Report
Computed Tomography Systems Pipeline Market Segments Outlook
- 64-slice CT Systems
- 16-slice and below CT Systems
Computed Tomography Systems Pipeline Market Territories Outlook
- The US
- Europe
- China
- Japan
- Australia
Computed Tomography Systems Pipeline Market Regulatory Paths Outlook
- 510(k)
- NMPA
- CE
- UKCA
- TGA
Scope
- Extensive coverage of the computed tomography systems under development
- Reviews details of major pipeline products which include, product description, licensing and collaboration details, and other developmental activities
- Reviews the major players involved in the development of computed tomography systems and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of computed tomography systems under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Annalise-AI Pty Ltd
Bioptics Technology LLC
California Institute of Technology
Canon Medical Systems Corp
Canon Medical Systems USA Inc
Duke University
Fermilab
FMI Medical Systems Inc
GE HealthCare Technologies Inc
German Cancer Research Center
Hitachi Medical Corp
Imatrex Inc
Izotropic Corp
Johns Hopkins University
Koning Corp
Koninklijke Philips NV
Malcova LLC
Massachusetts General Hospital
Micro-X Ltd
Nano Vision
Nanovision Technology Beijing Co Ltd
National Institutes of Health Clinical Center
NeuralRad LLC
Neusoft Medical Systems Co Ltd
Norsk Luftambulanse AS
Philips Healthcare
Shanghai United Imaging Healthcare Co Ltd
Siemens Healthineers AG
Stanford University
Stellarray, Inc.
TetraImaging LLC
The Hong Kong University of Science and Technology
The University of Manchester
Tribogenics, Inc.
United Imaging Systems (Beijing) Co Ltd
University Health Network
University of Antwerp
University of Auckland
University of California Santa Cruz
University of Louisville
University of Washington
Varex Imaging Corp
Varian Medical Systems Inc
Xoran Technologies LLC
ZumaTek, Inc.
Table of Contents
Table
Figures
Frequently asked questions
-
Which segment has the highest number of pipeline products in the computed tomography systems pipeline market as of May 2023?
As of May 2023, 64-slice CT systems have the highest number of pipeline products.
-
Which territory has the highest number of pipeline products in the computed tomography systems pipeline market as of May 2023?
As of May 2023, the US has the highest number of pipeline products in the computed tomography systems pipeline market.
-
Which is the most followed regulatory pathway in the computed tomography systems pipeline market As of May 2023?
As of May 2023, 510(k) is the most followed regulatory pathway in the computed tomography systems pipeline market.
-
Who are the major players operating in the computed tomography systems pipeline market?
Some of the major players operating in the computed tomography systems pipeline market are Advanced Breast-CT GmbH, Annalise-AI Pty Ltd, Bioptics Technology LLC, California Institute of Technology, Canon Medical Systems Corp, and Canon Medical Systems USA Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Computed Tomography (CT) Systems reports