1 Table of Contents 3
1.1 List of Tables 11
1.2 List of Figures 20
2 Introduction 21
2.1 Ventilators Overview 21
3 Products under Development 22
3.1 Ventilators – Pipeline Products by Stage of Development 22
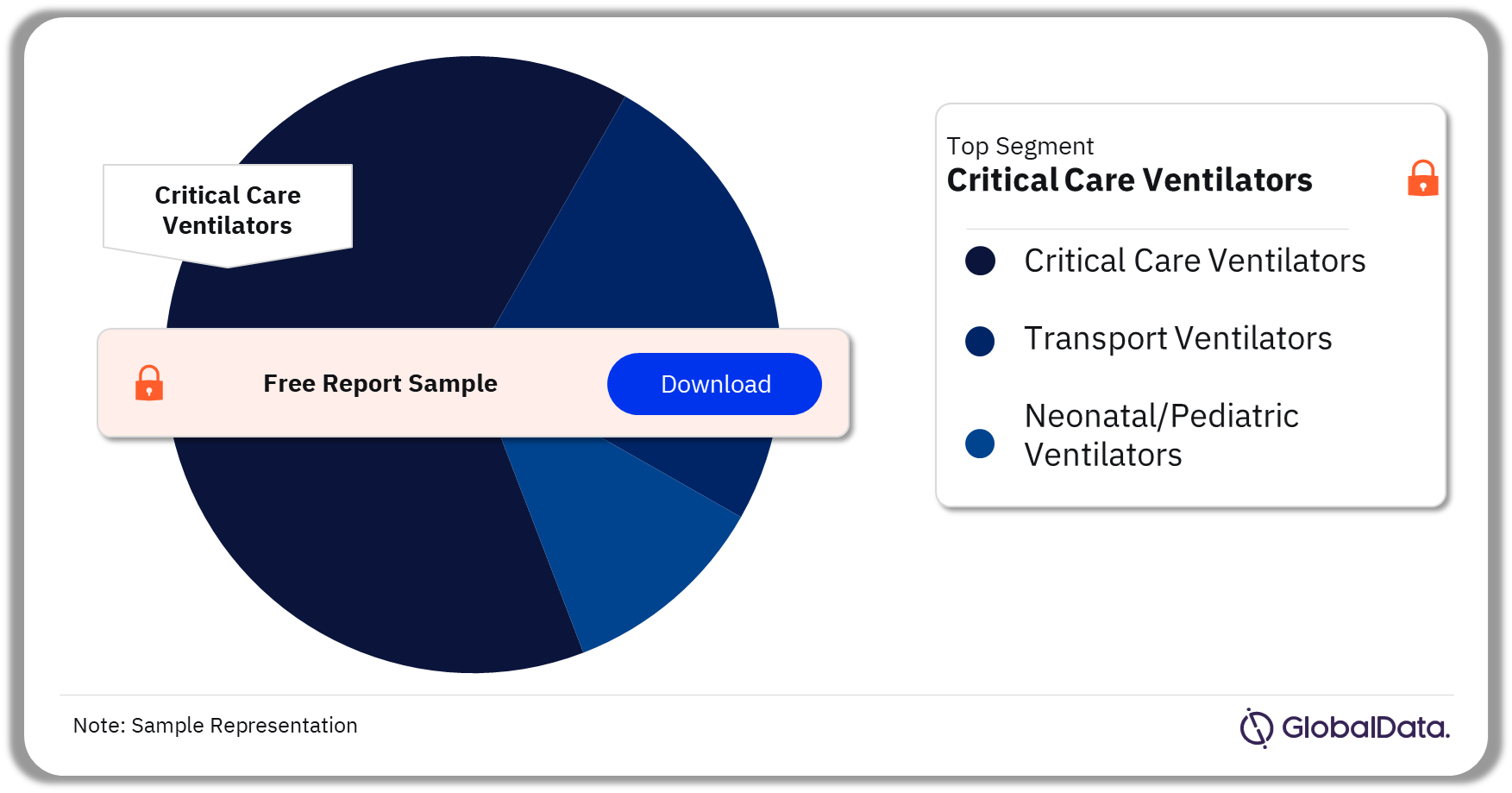
3.2 Ventilators – Pipeline Products by Segment 23
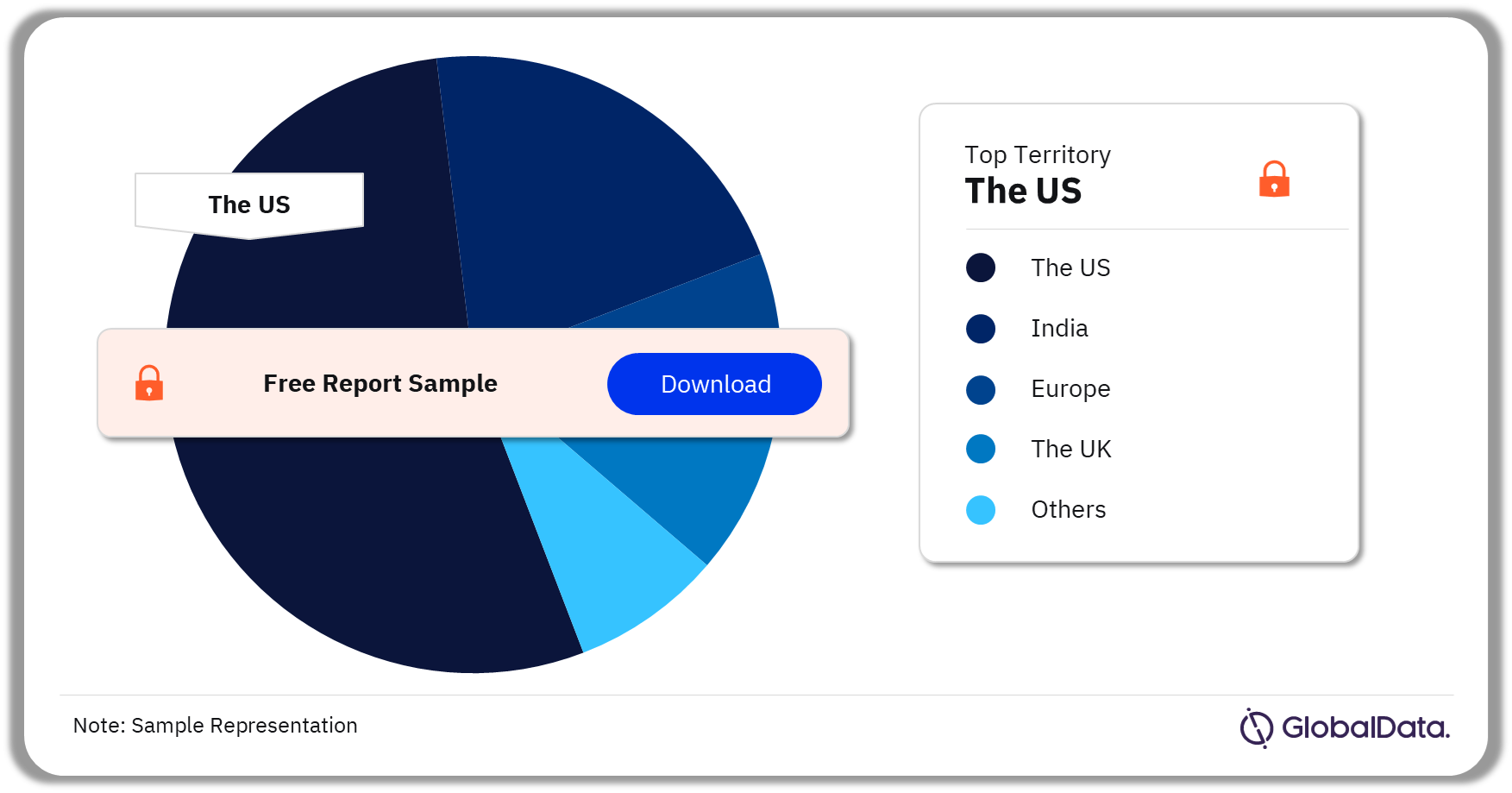
3.3 Ventilators – Pipeline Products by Territory 24
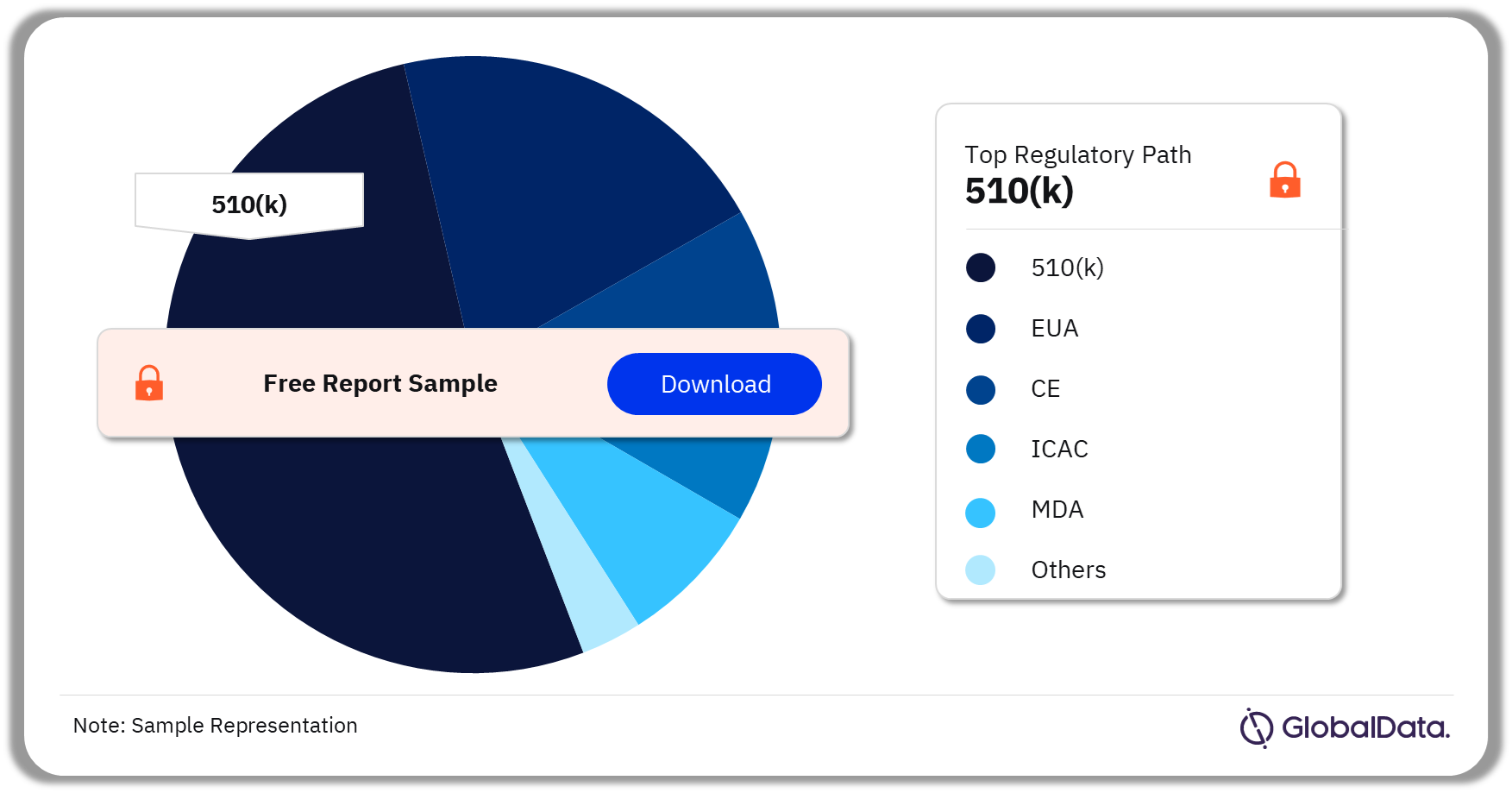
3.4 Ventilators – Pipeline Products by Regulatory Path 26
3.5 Ventilators – Pipeline Products by Estimated Approval Date 27
3.6 Ventilators – Ongoing Clinical Trials 28
4 Ventilators – Pipeline Products under Development by Companies 29
4.1 Ventilators Companies – Pipeline Products by Stage of Development 29
4.2 Ventilators – Pipeline Products by Stage of Development 35
5 Ventilators Companies and Product Overview 39
5.1 ABM Respiratory Care Company Overview 39
5.1.1 ABM Respiratory Care Pipeline Products & Ongoing Clinical Trials Overview 39
5.2 Aerobiosys innovations Pvt Ltd Company Overview 40
5.2.1 Aerobiosys innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 40
5.3 Aetherus BV Company Overview 41
5.3.1 Aetherus BV Pipeline Products & Ongoing Clinical Trials Overview 41
5.4 AgVa Healthcare Pvt Ltd Company Overview 42
5.4.1 AgVa Healthcare Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 42
5.5 Air Liquide Medical Systems SA Company Overview 44
5.5.1 Air Liquide Medical Systems SA Pipeline Products & Ongoing Clinical Trials Overview 44
5.6 All India Institute of Medical Sciences Company Overview 45
5.6.1 All India Institute of Medical Sciences Pipeline Products & Ongoing Clinical Trials Overview 45
5.7 ArcelorMittal India Pvt Ltd Company Overview 46
5.7.1 ArcelorMittal India Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 46
5.8 Armadilla Ltd Company Overview 47
5.8.1 Armadilla Ltd Pipeline Products & Ongoing Clinical Trials Overview 47
5.9 Ashok Leyland Ltd Company Overview 48
5.9.1 Ashok Leyland Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
5.10 Babcock International Group Plc Company Overview 49
5.10.1 Babcock International Group Plc Pipeline Products & Ongoing Clinical Trials Overview 49
5.11 Baxter Academy for Technology and Science Company Overview 50
5.11.1 Baxter Academy for Technology and Science Pipeline Products & Ongoing Clinical Trials Overview 50
5.12 Ben-Gurion University of the Negev Company Overview 51
5.12.1 Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 51
5.13 Bessel LLC Company Overview 52
5.13.1 Bessel LLC Pipeline Products & Ongoing Clinical Trials Overview 52
5.14 Bhagwati Products Ltd Company Overview 53
5.14.1 Bhagwati Products Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
5.15 breathe medical AG Company Overview 54
5.15.1 breathe medical AG Pipeline Products & Ongoing Clinical Trials Overview 54
5.16 Cambridge Consultants Ltd Company Overview 55
5.16.1 Cambridge Consultants Ltd Pipeline Products & Ongoing Clinical Trials Overview 55
5.17 Carlos III University of Madrid Company Overview 56
5.17.1 Carlos III University of Madrid Pipeline Products & Ongoing Clinical Trials Overview 56
5.18 Certus Critical Care Inc Company Overview 57
5.18.1 Certus Critical Care Inc Pipeline Products & Ongoing Clinical Trials Overview 57
5.19 Cionic Inc Company Overview 58
5.19.1 Cionic Inc Pipeline Products & Ongoing Clinical Trials Overview 58
5.20 Columbia University Company Overview 59
5.20.1 Columbia University Pipeline Products & Ongoing Clinical Trials Overview 59
5.21 ConzumeX Industries Pvt Ltd Company Overview 60
5.21.1 ConzumeX Industries Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 60
5.22 Cubic Corp Company Overview 62
5.22.1 Cubic Corp Pipeline Products & Ongoing Clinical Trials Overview 62
5.23 Don Bosco Technical College Company Overview 63
5.23.1 Don Bosco Technical College Pipeline Products & Ongoing Clinical Trials Overview 63
5.24 Dyson Ltd Company Overview 64
5.24.1 Dyson Ltd Pipeline Products & Ongoing Clinical Trials Overview 64
5.25 European Organization for Nuclear Research Company Overview 65
5.25.1 European Organization for Nuclear Research Pipeline Products & Ongoing Clinical Trials Overview 65
5.26 Ezz Medical Industries Co Company Overview 66
5.26.1 Ezz Medical Industries Co Pipeline Products & Ongoing Clinical Trials Overview 66
5.27 First Wave Technologies Inc Company Overview 69
5.27.1 First Wave Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 69
5.28 Formlabs Inc Company Overview 70
5.28.1 Formlabs Inc Pipeline Products & Ongoing Clinical Trials Overview 70
5.29 Foundry Medical Technologies Pvt Ltd Company Overview 71
5.29.1 Foundry Medical Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 71
5.30 Gas N2itrogen SL Company Overview 72
5.30.1 Gas N2itrogen SL Pipeline Products & Ongoing Clinical Trials Overview 72
5.31 GE HealthCare Technologies Inc Company Overview 73
5.31.1 GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 73
5.32 Georgia Institute of Technology Company Overview 74
5.32.1 Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 74
5.33 Gilero LLC Company Overview 75
5.33.1 Gilero LLC Pipeline Products & Ongoing Clinical Trials Overview 75
5.34 Griffith University Company Overview 76
5.34.1 Griffith University Pipeline Products & Ongoing Clinical Trials Overview 76
5.35 Hamilton Medical AG Company Overview 77
5.35.1 Hamilton Medical AG Pipeline Products & Ongoing Clinical Trials Overview 77
5.36 Imperial College London Company Overview 78
5.36.1 Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 78
5.37 Inali Foundation Company Overview 79
5.37.1 Inali Foundation Pipeline Products & Ongoing Clinical Trials Overview 79
5.38 Inbentus Company Overview 80
5.38.1 Inbentus Pipeline Products & Ongoing Clinical Trials Overview 80
5.39 Indian Institute of Science Company Overview 82
5.39.1 Indian Institute of Science Pipeline Products & Ongoing Clinical Trials Overview 82
5.40 Indian Institute of Technology Bhubaneswar Company Overview 83
5.40.1 Indian Institute of Technology Bhubaneswar Pipeline Products & Ongoing Clinical Trials Overview 83
5.41 Indian Institute of Technology Bombay Company Overview 85
5.41.1 Indian Institute of Technology Bombay Pipeline Products & Ongoing Clinical Trials Overview 85
5.42 Indian Institute of Technology Delhi Company Overview 87
5.42.1 Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 87
5.43 Indian Institute of Technology Dhanbad Company Overview 88
5.43.1 Indian Institute of Technology Dhanbad Pipeline Products & Ongoing Clinical Trials Overview 88
5.44 Indian Institute of Technology Guwahati Company Overview 89
5.44.1 Indian Institute of Technology Guwahati Pipeline Products & Ongoing Clinical Trials Overview 89
5.45 Indian Institute of Technology Hyderabad Company Overview 90
5.45.1 Indian Institute of Technology Hyderabad Pipeline Products & Ongoing Clinical Trials Overview 90
5.46 Indian Institute of Technology Jammu Company Overview 91
5.46.1 Indian Institute of Technology Jammu Pipeline Products & Ongoing Clinical Trials Overview 91
5.47 Indian Institute of Technology Kharagpur Company Overview 92
5.47.1 Indian Institute of Technology Kharagpur Pipeline Products & Ongoing Clinical Trials Overview 92
5.48 Indian Institute of Technology Palakkad Company Overview 93
5.48.1 Indian Institute of Technology Palakkad Pipeline Products & Ongoing Clinical Trials Overview 93
5.49 Indian Institute of Technology Roorkee Company Overview 94
5.49.1 Indian Institute of Technology Roorkee Pipeline Products & Ongoing Clinical Trials Overview 94
5.50 Indian Institute of Technology Ropar Company Overview 95
5.50.1 Indian Institute of Technology Ropar Pipeline Products & Ongoing Clinical Trials Overview 95
5.51 Indian Institute of Technology Tirupati Company Overview 96
5.51.1 Indian Institute of Technology Tirupati Pipeline Products & Ongoing Clinical Trials Overview 96
5.52 Inogen Inc Company Overview 97
5.52.1 Inogen Inc Pipeline Products & Ongoing Clinical Trials Overview 97
5.53 Integrated Polytechnic Regional College Kigali Company Overview 98
5.53.1 Integrated Polytechnic Regional College Kigali Pipeline Products & Ongoing Clinical Trials Overview 98
5.54 Johns Hopkins University Company Overview 99
5.54.1 Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 99
5.55 Kapurthala Railway Coach Factory Company Overview 100
5.55.1 Kapurthala Railway Coach Factory Pipeline Products & Ongoing Clinical Trials Overview 100
5.56 Khalifa University Company Overview 101
5.56.1 Khalifa University Pipeline Products & Ongoing Clinical Trials Overview 101
5.57 Kiira Motors Corp Company Overview 102
5.57.1 Kiira Motors Corp Pipeline Products & Ongoing Clinical Trials Overview 102
5.58 K-One Technology Berhad Company Overview 103
5.58.1 K-One Technology Berhad Pipeline Products & Ongoing Clinical Trials Overview 103
5.59 Kreator 3d Printer Solutions Pvt Ltd Company Overview 104
5.59.1 Kreator 3d Printer Solutions Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 104
5.60 Kritikare India Pvt Ltd Company Overview 105
5.60.1 Kritikare India Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 105
5.61 Lawrence Livermore National Laboratory Company Overview 106
5.61.1 Lawrence Livermore National Laboratory Pipeline Products & Ongoing Clinical Trials Overview 106
5.62 LifeCan Medical Ltd Company Overview 107
5.62.1 LifeCan Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 107
5.63 Ligand Innovation Global Inc Company Overview 108
5.63.1 Ligand Innovation Global Inc Pipeline Products & Ongoing Clinical Trials Overview 108
5.64 Lund University Company Overview 109
5.64.1 Lund University Pipeline Products & Ongoing Clinical Trials Overview 109
5.65 Mahindra & Mahindra Ltd Company Overview 110
5.65.1 Mahindra & Mahindra Ltd Pipeline Products & Ongoing Clinical Trials Overview 110
5.66 Massachusetts Institute of Technology Company Overview 112
5.66.1 Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 112
5.67 Mediklik Webhealth Pvt Ltd Company Overview 115
5.67.1 Mediklik Webhealth Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 115
5.68 Mergenet Medical, Inc. Company Overview 116
5.68.1 Mergenet Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 116
5.69 MFM LLC Company Overview 117
5.69.1 MFM LLC Pipeline Products & Ongoing Clinical Trials Overview 117
5.70 MG Motor India Pvt Ltd Company Overview 118
5.70.1 MG Motor India Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 118
5.71 Michigan Critical Care Consultants Inc Company Overview 119
5.71.1 Michigan Critical Care Consultants Inc Pipeline Products & Ongoing Clinical Trials Overview 119
5.72 Michigan State University Company Overview 120
5.72.1 Michigan State University Pipeline Products & Ongoing Clinical Trials Overview 120
5.73 Ministry of Energy and Mineral Resources, Indonesia Company Overview 121
5.73.1 Ministry of Energy and Mineral Resources, Indonesia Pipeline Products & Ongoing Clinical Trials Overview 121
5.74 Monivent AB Company Overview 122
5.74.1 Monivent AB Pipeline Products & Ongoing Clinical Trials Overview 122
5.75 Mullen Technologies Inc (Inactive) (Inactive) Company Overview 123
5.75.1 Mullen Technologies Inc (Inactive) (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 123
5.76 National Institute of Technology Durgapur Company Overview 124
5.76.1 National Institute of Technology Durgapur Pipeline Products & Ongoing Clinical Trials Overview 124
5.77 Naval Sea Logistics Center Company Overview 125
5.77.1 Naval Sea Logistics Center Pipeline Products & Ongoing Clinical Trials Overview 125
5.78 Neonatal Rescue LLC Company Overview 126
5.78.1 Neonatal Rescue LLC Pipeline Products & Ongoing Clinical Trials Overview 126
5.79 Nihon Kohden Corp Company Overview 127
5.79.1 Nihon Kohden Corp Pipeline Products & Ongoing Clinical Trials Overview 127
5.80 Nocca Robotics Pvt Ltd Company Overview 128
5.80.1 Nocca Robotics Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 128
5.81 Nova Scotia Health Authority Company Overview 129
5.81.1 Nova Scotia Health Authority Pipeline Products & Ongoing Clinical Trials Overview 129
5.82 NumaVent Company Overview 130
5.82.1 NumaVent Pipeline Products & Ongoing Clinical Trials Overview 130
5.83 NVIDIA Corp Company Overview 131
5.83.1 NVIDIA Corp Pipeline Products & Ongoing Clinical Trials Overview 131
5.84 OneBreath Inc. Company Overview 132
5.84.1 OneBreath Inc. Pipeline Products & Ongoing Clinical Trials Overview 132
5.85 Oregon Health & Science University Company Overview 133
5.85.1 Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 133
5.86 Orixha Company Overview 134
5.86.1 Orixha Pipeline Products & Ongoing Clinical Trials Overview 134
5.87 OscillaVent Inc Company Overview 135
5.87.1 OscillaVent Inc Pipeline Products & Ongoing Clinical Trials Overview 135
5.88 Padmaseetha Technologies Pvt Ltd Company Overview 136
5.88.1 Padmaseetha Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 136
5.89 Philips Healthcare Informatics Inc Company Overview 137
5.89.1 Philips Healthcare Informatics Inc Pipeline Products & Ongoing Clinical Trials Overview 137
5.90 Polytechnic University of Valencia Company Overview 138
5.90.1 Polytechnic University of Valencia Pipeline Products & Ongoing Clinical Trials Overview 138
5.91 Quantaira Health Company Overview 139
5.91.1 Quantaira Health Pipeline Products & Ongoing Clinical Trials Overview 139
5.92 Rathinam Group of Institutions Company Overview 140
5.92.1 Rathinam Group of Institutions Pipeline Products & Ongoing Clinical Trials Overview 140
5.93 Rethink Respironics Inc Company Overview 141
5.93.1 Rethink Respironics Inc Pipeline Products & Ongoing Clinical Trials Overview 141
5.94 Rethink Technologies LLC Company Overview 142
5.94.1 Rethink Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 142
5.95 RightAir LLC Company Overview 143
5.95.1 RightAir LLC Pipeline Products & Ongoing Clinical Trials Overview 143
5.96 Sagentia Ltd Company Overview 144
5.96.1 Sagentia Ltd Pipeline Products & Ongoing Clinical Trials Overview 144
5.97 SaiOx Inc Company Overview 145
5.97.1 SaiOx Inc Pipeline Products & Ongoing Clinical Trials Overview 145
5.98 SLE Ltd Company Overview 146
5.98.1 SLE Ltd Pipeline Products & Ongoing Clinical Trials Overview 146
5.99 Smith & Nephew Plc Company Overview 149
5.99.1 Smith & Nephew Plc Pipeline Products & Ongoing Clinical Trials Overview 149
5.100 Smith College Company Overview 150
5.100.1 Smith College Pipeline Products & Ongoing Clinical Trials Overview 150
5.101 Southern Railway Company Overview 151
5.101.1 Southern Railway Pipeline Products & Ongoing Clinical Trials Overview 151
5.102 Sree Chitra Tirunal Institute for Medical Sciences & Technology Company Overview 152
5.102.1 Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 152
5.103 Staffordshire University Company Overview 153
5.103.1 Staffordshire University Pipeline Products & Ongoing Clinical Trials Overview 153
5.104 Stephan Design and Engineering Ltd Company Overview 154
5.104.1 Stephan Design and Engineering Ltd Pipeline Products & Ongoing Clinical Trials Overview 154
5.105 Steros GPA Innovative SL Company Overview 155
5.105.1 Steros GPA Innovative SL Pipeline Products & Ongoing Clinical Trials Overview 155
5.106 Stogger BV Company Overview 156
5.106.1 Stogger BV Pipeline Products & Ongoing Clinical Trials Overview 156
5.107 The Chaim Sheba Medical Center Company Overview 157
5.107.1 The Chaim Sheba Medical Center Pipeline Products & Ongoing Clinical Trials Overview 157
5.108 The Lundquist Institute Company Overview 158
5.108.1 The Lundquist Institute Pipeline Products & Ongoing Clinical Trials Overview 158
5.109 TKM College of Engineering-Kerala University Company Overview 159
5.109.1 TKM College of Engineering-Kerala University Pipeline Products & Ongoing Clinical Trials Overview 159
5.110 Tolomatic Inc Company Overview 160
5.110.1 Tolomatic Inc Pipeline Products & Ongoing Clinical Trials Overview 160
5.111 Toyota Motor Corp Company Overview 161
5.111.1 Toyota Motor Corp Pipeline Products & Ongoing Clinical Trials Overview 161
5.112 TVP Health Company Overview 162
5.112.1 TVP Health Pipeline Products & Ongoing Clinical Trials Overview 162
5.113 Universidad Nacional de Colombia Company Overview 163
5.113.1 Universidad Nacional de Colombia Pipeline Products & Ongoing Clinical Trials Overview 163
5.114 Universiti Teknologi MARA Company Overview 164
5.114.1 Universiti Teknologi MARA Pipeline Products & Ongoing Clinical Trials Overview 164
5.115 University College Dublin Company Overview 165
5.115.1 University College Dublin Pipeline Products & Ongoing Clinical Trials Overview 165
5.116 University of Aberdeen Company Overview 166
5.116.1 University of Aberdeen Pipeline Products & Ongoing Clinical Trials Overview 166
5.117 University of Antioquia Company Overview 167
5.117.1 University of Antioquia Pipeline Products & Ongoing Clinical Trials Overview 167
5.118 University of Arizona Company Overview 168
5.118.1 University of Arizona Pipeline Products & Ongoing Clinical Trials Overview 168
5.119 University of Barcelona Company Overview 170
5.119.1 University of Barcelona Pipeline Products & Ongoing Clinical Trials Overview 170
5.120 University of Botswana Company Overview 171
5.120.1 University of Botswana Pipeline Products & Ongoing Clinical Trials Overview 171
5.121 University of California Los Angeles Company Overview 172
5.121.1 University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 172
5.122 University of California San Diego Company Overview 173
5.122.1 University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 173
5.123 University of California San Francisco Company Overview 175
5.123.1 University of California San Francisco Pipeline Products & Ongoing Clinical Trials Overview 175
5.124 University of Connecticut Company Overview 176
5.124.1 University of Connecticut Pipeline Products & Ongoing Clinical Trials Overview 176
5.125 University of Florida Company Overview 177
5.125.1 University of Florida Pipeline Products & Ongoing Clinical Trials Overview 177
5.126 University of South Florida Company Overview 178
5.126.1 University of South Florida Pipeline Products & Ongoing Clinical Trials Overview 178
5.127 University of Texas at San Antonio Company Overview 180
5.127.1 University of Texas at San Antonio Pipeline Products & Ongoing Clinical Trials Overview 180
5.128 University of Utah Company Overview 181
5.128.1 University of Utah Pipeline Products & Ongoing Clinical Trials Overview 181
5.129 University of Warwick Company Overview 182
5.129.1 University of Warwick Pipeline Products & Ongoing Clinical Trials Overview 182
5.130 Vapotherm Inc Company Overview 183
5.130.1 Vapotherm Inc Pipeline Products & Ongoing Clinical Trials Overview 183
5.131 Ventec Life Systems Inc Company Overview 184
5.131.1 Ventec Life Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 184
5.132 Ventis Medical Inc Company Overview 186
5.132.1 Ventis Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 186
5.133 Vexos Corp Company Overview 187
5.133.1 Vexos Corp Pipeline Products & Ongoing Clinical Trials Overview 187
5.134 Villanova University Company Overview 188
5.134.1 Villanova University Pipeline Products & Ongoing Clinical Trials Overview 188
5.135 Vrije University Brussel Company Overview 189
5.135.1 Vrije University Brussel Pipeline Products & Ongoing Clinical Trials Overview 189
5.136 Walter Reed National Military Medical Center Company Overview 190
5.136.1 Walter Reed National Military Medical Center Pipeline Products & Ongoing Clinical Trials Overview 190
5.137 Wise Ally International Holdings Ltd Company Overview 191
5.137.1 Wise Ally International Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 191
5.138 Worcester Polytechnic Institute Company Overview 192
5.138.1 Worcester Polytechnic Institute Pipeline Products & Ongoing Clinical Trials Overview 192
5.139 X-Biomedical Inc Company Overview 193
5.139.1 X-Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 193
5.140 Zen Technologies Ltd Company Overview 194
5.140.1 Zen Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 194
6 Ventilators- Recent Developments 195
6.1 Mar 19, 2024: Fisher & Paykel Board Chair to Retire in August 195
6.2 Mar 15, 2024: Vapotherm to Unveil Access365 Home Ventilation Solution at MEDTRADE 195
6.3 Mar 12, 2024: Medtronic To Present At Barclays Global Healthcare Conference 195
6.4 Mar 08, 2024: Nihon Kohden America Receives Additional FDA 510(k) Clearance for NKV-550 Series Ventilator System 195
6.5 Mar 08, 2024: Fisher & Paykel Healthcare Board Chair Scott St John to Retire in 2024 196
6.6 Mar 07, 2024: Ventec Life Systems Recalls VOCSN Patient Breathing Package (Pediatric, Active, Oxygen, Blue) for Manufacturing Issue with Bonded Spiral Wrap 196
6.7 Feb 28, 2024: Bob Azelby to Join Cardinal Health Board of Directors 196
6.8 Feb 20, 2024: Medtronic Announces Ventilator Business Changes: February 2024 197
6.9 Feb 14, 2024: GE HealthCare Management to Present at Upcoming Investor Conferences 197
6.10 Feb 08, 2024: Teleflex Announces Fourth Quarter 2023 Earnings Conference Call Information 197
6.11 Feb 06, 2024: Getinge’s ventilators get a virtual twin 198
6.12 Feb 06, 2024: INSERTING and REPLACING GE HealthCare Reports Fourth Quarter and Full Year 2023 Financial Results 198
6.13 Jan 25, 2024: Philips Respironics Announces Sleep & Respiratory Product Portfolio Changes 203
6.14 Jan 23, 2024: Agneta Palmer Appointed New CFO of Getinge 207
6.15 Jan 18, 2024: GE HealthCare to Announce Fourth Quarter and Full Year 2023 Results on February 6, 2024 208
6.16 Dec 22, 2023: Newegg Commerce Reports Earnings Results for the Nine Months Ended September 30, 2023 208
6.17 Dec 19, 2023: Fisher & Paykel Healthcare Announces Board Changes 208
6.18 Dec 06, 2023: Medical Technology Innovator Thornhill Medical’s MOVES SLC Is Acquired for United States Marine Corp En Route Care Modernization and Added to Authorized Medical Allowance List (AMAL) 647 208
6.19 Dec 04, 2023: Thornhill Medical Teams up with Leading Medical Solutions Providers to Develop Medical Surge Capabilities for U.S. National Disaster Medical System 209
6.20 Nov 28, 2023: Thornhill Medical’s MOVES SLC deployed as part of the first En-Route Care System (ERCS) aboard the U.S. Navy’s Eisenhower Strike Group (IKECSG) 210
6.21 Nov 20, 2023: Molecule Holdings Announces Change of Chief Financial Officer 210
6.22 Nov 17, 2023: Ambu Notice for the Annual General Meeting 211
6.23 Nov 05, 2023: Teleflex Reports Third Quarter Financial Results, Full Year 2023 Outlook 211
6.24 Nov 03, 2023: Cardinal Health Reports First Quarter Fiscal 2024 Results and Raises Fiscal 2024 Outlook 211
6.25 Nov 02, 2023: Inspiration Healthcare Group Announces Launch of SLE1500 Non-Invasive Ventilator 213
6.26 Oct 31, 2023: GE HealthCare Reports Third Quarter 2023 Financial Results 213
6.27 Oct 26, 2023: ResMed Announces Results for the First Quarter of Fiscal Year 2024 215
6.28 Oct 25, 2023: Air Liquide Announces 2023 Third Quarter Revenue 216
6.29 Oct 23, 2023: Getinge Announces Interim Report July – September 2023 218
6.30 Oct 23, 2023: Getinge: Interim Report July – September 2023 219
6.31 Oct 20, 2023: Philips Respironics Recalls V60 and V60 Plus Ventilators Due to Power Management Printed Circuit Board Assemblies (PM Pcbas) Not Meeting Ventilator Standards 219
6.32 Oct 18, 2023: Hamilton Medical Recalls HAMILTON-C1, T1, MR-1 for Capacitator Leaks and Short Circuits 220
6.33 Oct 11, 2023: Ambu Appoints New Chief Financial Officer 221
6.34 Oct 09, 2023: Ambu Announces Preliminary Financial Results For The Fiscal Year 2022/23 222
6.35 Oct 04, 2023: Medtronic Names Paolo Di Vincenzo President of the Neuromodulation Business 222
6.36 Oct 03, 2023: Getinge Invites Fund Managers, Analysts, and Media to Q3 Report 2023 Conference Call 223
6.37 Sep 12, 2023: Air Liquide Appoints CEO of the Americas hub 223
6.38 Sep 07, 2023: Villanova Engineers Awarded Patent for Low-Cost NovaVent Mechanical Ventilator 223
6.39 Sep 07, 2023: Philips Reaches Resolution of US Economic Loss Litigation 224
6.40 Sep 05, 2023: ResMed Announces Participation in Upcoming Conferences 225
6.41 Aug 30, 2023: BioLineRx Reports Second Quarter 2023 Financial Results and Recent Corporate and Portfolio Updates 225
6.42 Aug 29, 2023: F&P Healthcare Flags 14% Revenue Growth in First Half 227
6.43 Aug 29, 2023: Newegg Announces First Half 2023 Results 227
6.44 Aug 26, 2023: Medtronic Q2 2024 Earnings Estimates Increased by William Blair 228
6.45 Aug 24, 2023: OSI Systems Posts Results for Q4 and Fiscal Year Ended June 30, 2023 228
6.46 Aug 23, 2023: Draeger Recalls Carina Sub-Acute Care Ventilators for Contaminants in Airpath 229
6.47 Aug 15, 2023: Cardinal Health Reports Fourth Quarter and Full Year Results for Fiscal Year 2023 at High End of Investor Day Guidance 230
6.48 Aug 14, 2023: Philips Respironics Recalls Trilogy Evo, Evo O2, Ev300, And Evo Universal Ventilators After Finding Dust And Dirt In Air Path That Can Reduce Air Flow To Patients 231
6.49 Aug 08, 2023: Mindray and Operation Smile Begin 10-Year Partnership to Improve Healthcare for Vulnerable Communities 232
6.50 Aug 08, 2023: Medtronic to Announce Financial Results for its First Quarter of Fiscal Year 2024 233
6.51 Aug 03, 2023: XRP Healthcare and Spiritus Medical Partner to Revolutionize Healthcare in Africa 233
6.52 Aug 03, 2023: ResMed Announces Results for the Fourth Quarter of Fiscal Year 2023 234
6.53 Aug 03, 2023: Teleflex Reports Second Quarter Financial Results and Full Year 2023 Outlook 235
6.54 Jul 31, 2023: Sanmina Announces Third Quarter Fiscal 2023 Financial Results 239
6.55 Jul 28, 2023: Origin Medical Devices Receives 510(K) Clearance for Panther 5 Model P5DLVENT 240
6.56 Jul 27, 2023: Air Liquide Announces H1 2023: Solid Performance and Sustained Investment Momentum Paving the Way for the Future 240
6.57 Jul 27, 2023: Homology Lays off 87% of staff 244
6.58 Jul 24, 2023: Philips Delivers Improved Operational Performance Driven by Sales Growth and Focus on Execution 244
6.59 Jul 23, 2023: Fisher & Paykel Healthcare Announces Board Changes 247
6.60 Jul 19, 2023: ReddyPort non-invasive ventilation device receives FDA clearance 247
6.61 Jul 19, 2023: Baylis Medical Technologies Announces Delivery of 100 Baylis V4C-560 Ventilators to Aid Humanitarian Efforts in Ukraine 247
6.62 Jul 17, 2023: Resmed Receives 510(k) Clearance for Whitsundays Mask System 248
6.63 Jul 13, 2023: Draeger Medical Recalls Oxylog 3000 Plus Emergency and Transport Ventilators for Risk of Unexpected Depleted Battery and Ventilator Stop 248
6.64 Jul 07, 2023: Getinge Receives Us FDA 510(K) Clearance for Servo-Air Lite 249
6.65 Jul 05, 2023: Shenzhen Mindray Bio-Medical Electronics Receives 510(k) Clearance for SV600 Ventilator 249
6.66 Jul 05, 2023: Shenzhen Mindray Bio-Medical Electronics Receives 510(k) Clearance for SV800 Ventilator 249
6.67 Jun 29, 2023: Getinge invites fund managers, analysts, and media to Q2 Report 2023 conference call 250
6.68 Jun 19, 2023: Nihon Kohden OrangeMed Receives 510(k) Clearance for NKV-440 Ventilator System 250
6.69 Jun 13, 2023: Magnamed Tecnologia Medica Receives 510(k) Clearance for Oxymag Transport and Emergency Ventilator 250
6.70 May 30, 2023: Dragerwerk receives 510(k) clearance for Evita V800 250
6.71 May 30, 2023: Dragerwerk receives 510(k) clearance for Evita V600 250
6.72 May 30, 2023: Dragerwerk receives 510(k) clearance for Babylog VN600 Ventilator 251
6.73 May 30, 2023: Dragerwerk receives 510(k) clearance for Babylog VN800 251
6.74 May 30, 2023: BMC corporate launches new products and strategies at latest exhibitions 251
6.75 May 17, 2023: BioLineRx to report Q1 2023 results on May 24, 2023 252
6.76 May 16, 2023: Philips provides update on completed set of test results for CPAP/BiPAP sleep therapy devices 252
6.77 May 11, 2023: Medtronic to announce financial results for its fourth quarter and full fiscal year 2023 255
6.78 May 11, 2023: Sanmina posts results for fiscal Q2 ended April 1, 2023 255
6.79 May 11, 2023: Teijin Announces Consolidated Financial Statements Summary (For the year ended March 31, 2023) 257
6.80 May 09, 2023: GE HealthCare Names James Saccaro Vice President and Chief Financial Officer 258
6.81 May 08, 2023: Fisher & Paykel Healthcare to announce full year results on 26 May 2023 258
6.82 May 05, 2023: Drager’s Annual General Meeting approves stable dividend for fiscal year 2022 258
6.83 May 04, 2023: Teleflex reports first quarter financial results and full year 2023 outlook 259
6.84 May 04, 2023: Cardinal Health reports third quarter fiscal year 2023 results and raises fiscal year 2023 non-gaap eps guidance 261
6.85 May 02, 2023: ResMed Appoints Michael Rider its Next Global General Counsel, Dawn Haake its First Chief Quality Officer 263
6.86 May 01, 2023: Clinical Study Shows That Use of Monivent NEO100 Significantly Increases the Quality of Ventilation of Newborns 263
6.87 Apr 27, 2023: OSI Systems announces fiscal 2023 Q3 financial results 264
6.88 Apr 27, 2023: ResMed announces results for the third quarter of fiscal year 2023 266
6.89 Apr 26, 2023: Getinge announces interim report January-March 2023 268
6.90 Apr 26, 2023: Getinge announces resolutions of annual general Meeting 269
6.91 Apr 24, 2023: New clinical study demonstrates significant improvement in pulmonary health and related symptoms when using Provox Life 270
6.92 Apr 20, 2023: Teleflex announces first quarter 2023 earnings conference call information 271
6.93 Apr 12, 2023: Medtronic has a layoff in California 271
6.94 Apr 11, 2023: Getinge invites fund managers, analysts and media to Q1 Report 2023 conference call 271
6.95 Apr 06, 2023: ResMed to Report Third Quarter Fiscal 2023 Earnings on April 27, 2023 271
6.96 Apr 03, 2023: Getinge’s Announces Marlene and Dowreng featured on the cover of Annual Report 272
7 Appendix 273
7.1 Methodology 273
7.2 About GlobalData 276
7.3 Contact Us 276
7.4 Disclaimer 276
![]()