Endotracheal Tubes Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Endotracheal Tubes Pipeline Market Report Overview
Endotracheal tubes are used to maintain an unobstructed passageway especially to deliver oxygen or anesthesia to the lungs during surgery or any airway management procedure. The curved tube is placed through the patient’s nose or mouth into the trachea (windpipe).
The endotracheal tubes pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Territories | · United States
· Europe · Canada · Singapore · United Kingdom |
| Key Regulatory Paths | · 510(k)
· CE · EUA · HSA · MDL |
| Key Companies | · Allvivo Vascular Inc
· Avir Medical Pte Ltd · Baylor College of Medicine · Enox Biopharma Inc (Inactive) · Fogless International AB |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Endotracheal Tubes Pipeline Market Segmentation by Territories
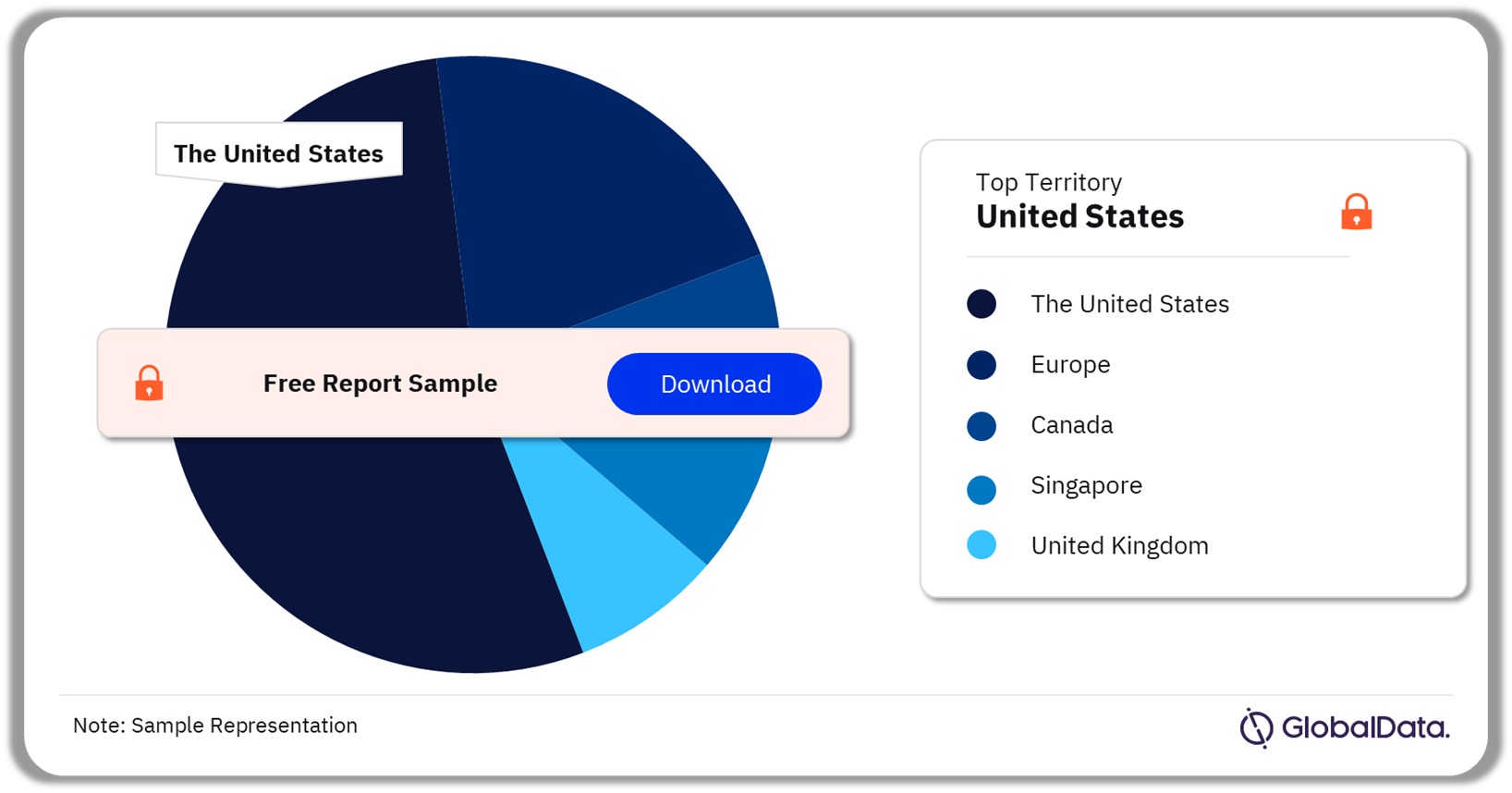
A few of the key territories for the endotracheal tube pipeline market are the United States, Europe, Canada, Singapore, and the United Kingdom. As of March 2024, the United States accounted for the highest number of pipeline products.
Endotracheal Tubes Pipeline Market Analysis by Territories, 2024 (%)
Buy the Full Report for More Territory Insights into the Endotracheal Tubes Pipeline Market
Endotracheal Tubes Pipeline Market Segmentation by Regulatory Paths
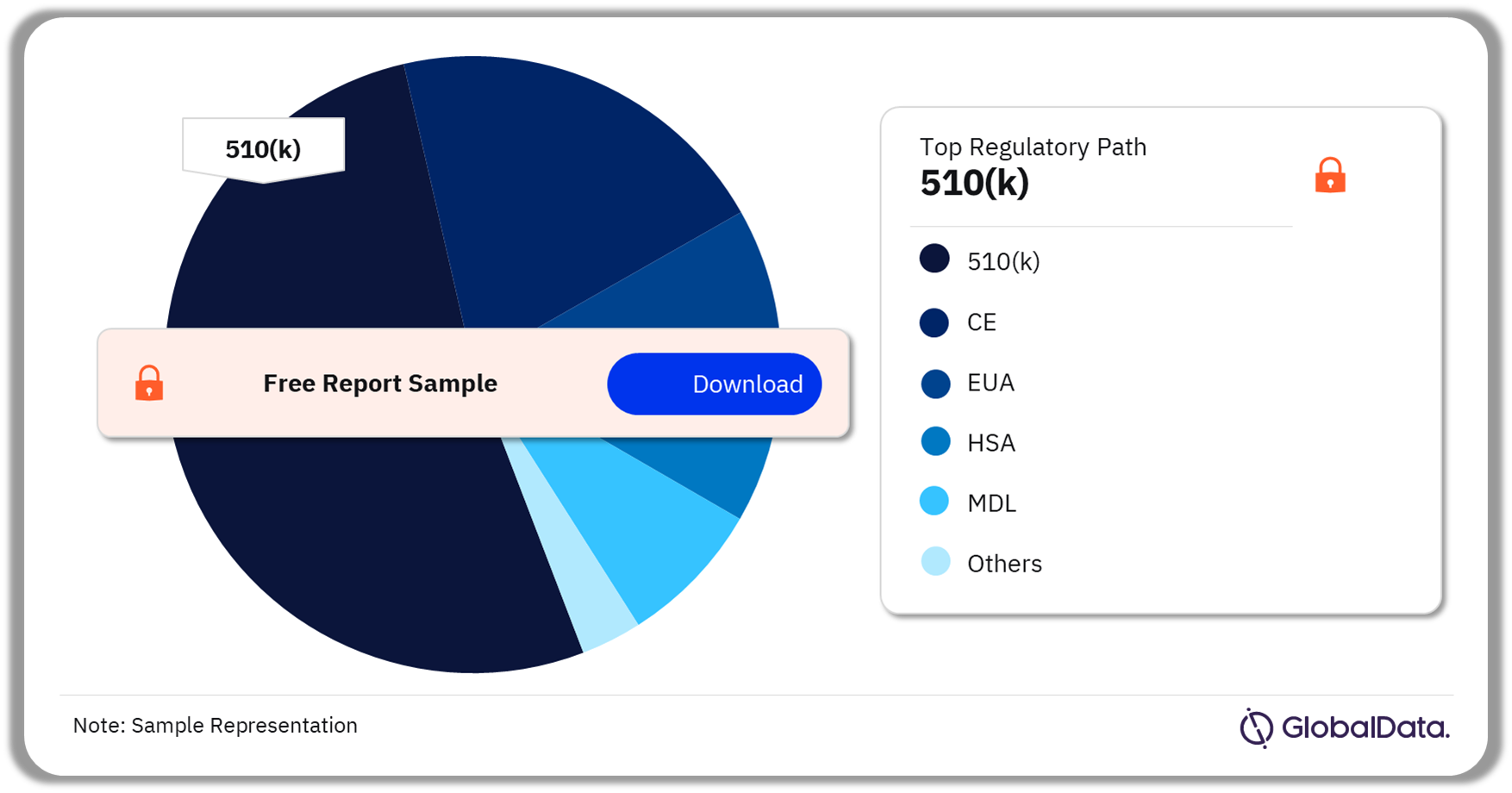
The key regulatory paths in the endotracheal tubes pipeline products market are 510(k), CE, EUA, HAS, MDL among others. As of March 2024, 510(k) was the most followed pathway for pipeline products in the market.
Endotracheal Tubes Pipeline Market Analysis by Regulatory Paths, 2024 (%)
Buy the Full Report for More Regulatory Path Insights into the Endotracheal Tubes Pipeline Market
Endotracheal Tubes Pipeline Market – Competitive Landscape
A few of the key companies associated with the endotracheal tubes pipeline market are Allvivo Vascular Inc, Avir Medical Pte Ltd, Baylor College of Medicine, Enox Biopharma Inc (Inactive), Fogless International AB among others.
Allvivo Vascular Inc: It is a medical technology company, which designs and develops biocompatible and antimicrobial coatings for medical devices and combination products. The company offers products that are based on Gatekeeper antimicrobial technology, which supports clients to practice responsible infection control. The company sells its products through a network of distributors across the US.
Baylor College of Medicine.: Baylor College of Medicine (BCM) is a health science university that offers educational services. It provides research, patient care, education, and community services. . The college offers various educational courses including M.D. Programs, Ph.D. programs, doctor of nurse practice, P.A. programs, dual degree programs, genetic counseling programs, orthotics and prosthetic programs.
Endotracheal Tubes Pipeline Market Analysis by Companies, 2024
Buy the Full Report for More Company Insights into the Endotracheal Tubes Pipeline Market
Segments Covered in the Report
Endotracheal Tubes Pipeline Market Territories Outlook
- United States
- Europe
- Canada
- Singapore
- United Kingdom
Endotracheal Tubes Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- EUA
- HSA
- MDL
Scope
This report provides:
- Extensive coverage of the endotracheal tubes under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of endotracheal tubes and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of endotracheal tubes under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out an in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Avir Medical Pte Ltd
Baylor College of Medicine
Enox Biopharma Inc (Inactive)
Fogless International AB
Iasis Molecular Sciences Inc
Illumitube
Johns Hopkins University
Laval University
Medical Device Investments, Inc.
N8 Medical LLC
P3 Medical Ltd
Resultados y Calidad del Sistema Sanitario Publico de Andalucia
Sharklet Technologies Inc
Sonoma Pharmaceuticals Inc
Sree Chitra Tirunal Institute for Medical Sciences & Technology
Teleflex Inc
The Chinese University of Hong Kong
University of Colorado
University of Illinois at Chicago
University of Michigan
University of Minnesota
University of Pittsburgh
University of Texas at San Antonio
University of Virginia
Virginia Commonwealth University
Vrije University Brussel
WynnVision LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory had the highest number of products in the endotracheal tubes pipeline market as of March 2024?
As of March 2024, the United States accounted for the highest number of pipeline products in the endotracheal tubes market.
-
Which was the most followed regulatory pathway in the endotracheal tubes pipeline market as of March 2024?
As of March 2024, 510(k) was the most followed pathway for pipeline products in the endotracheal tubes market.
-
Which are the major companies operating in the endotracheal tubes pipeline market?
A few of the key companies associated with the endotracheal tubes pipeline market are Allvivo Vascular Inc, Avir Medical Pte Ltd, Baylor College of Medicine, Enox Biopharma Inc (Inactive), Fogless International AB among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Endotracheal Tubes reports