Cystic Fibrosis – Global Drug Forecast and Market Analysis to 2030
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
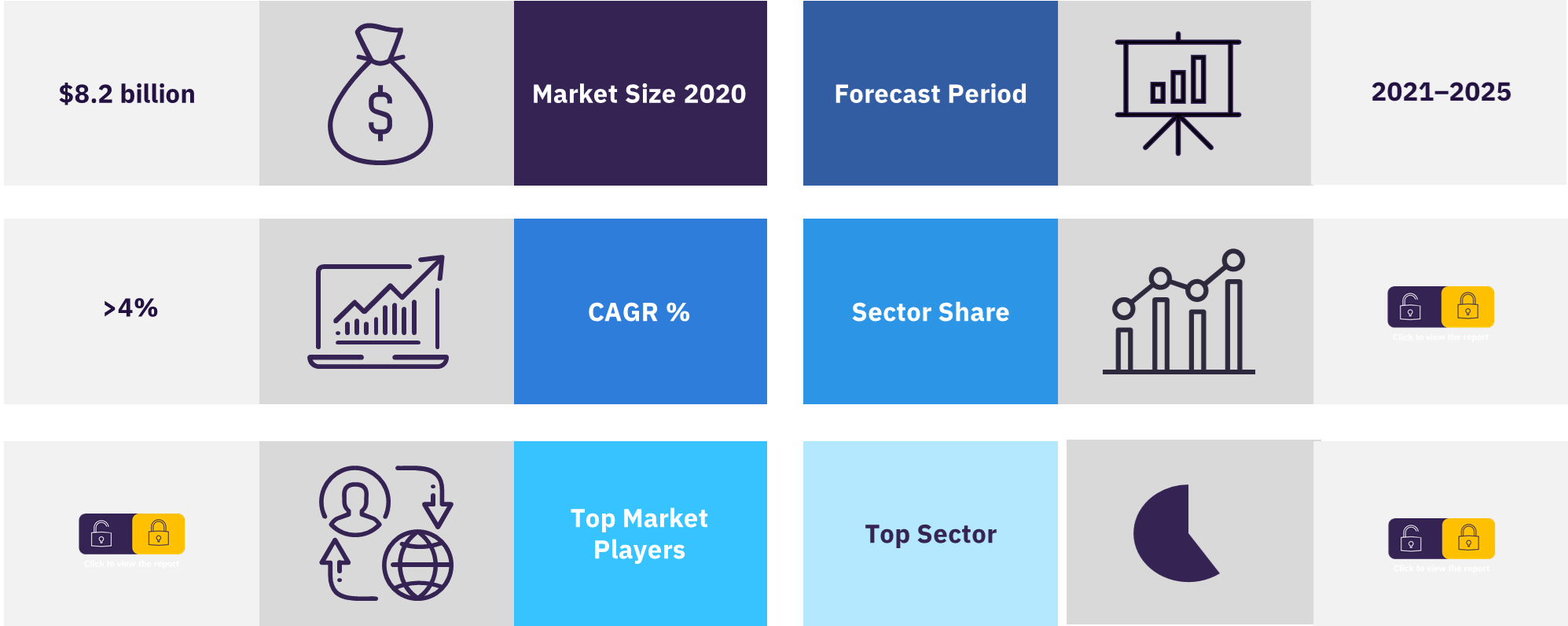
The global (7MM) sales for the cystic fibrosis (CF) have been valued at $8.2 billion in 2020. The market is expected to grow at a CAGR of more than 4% during 2021-2025. Over the past decade, there have been significant changes in the CF market due to the development of CFTR modulator therapies that target the underlying cause of CF.
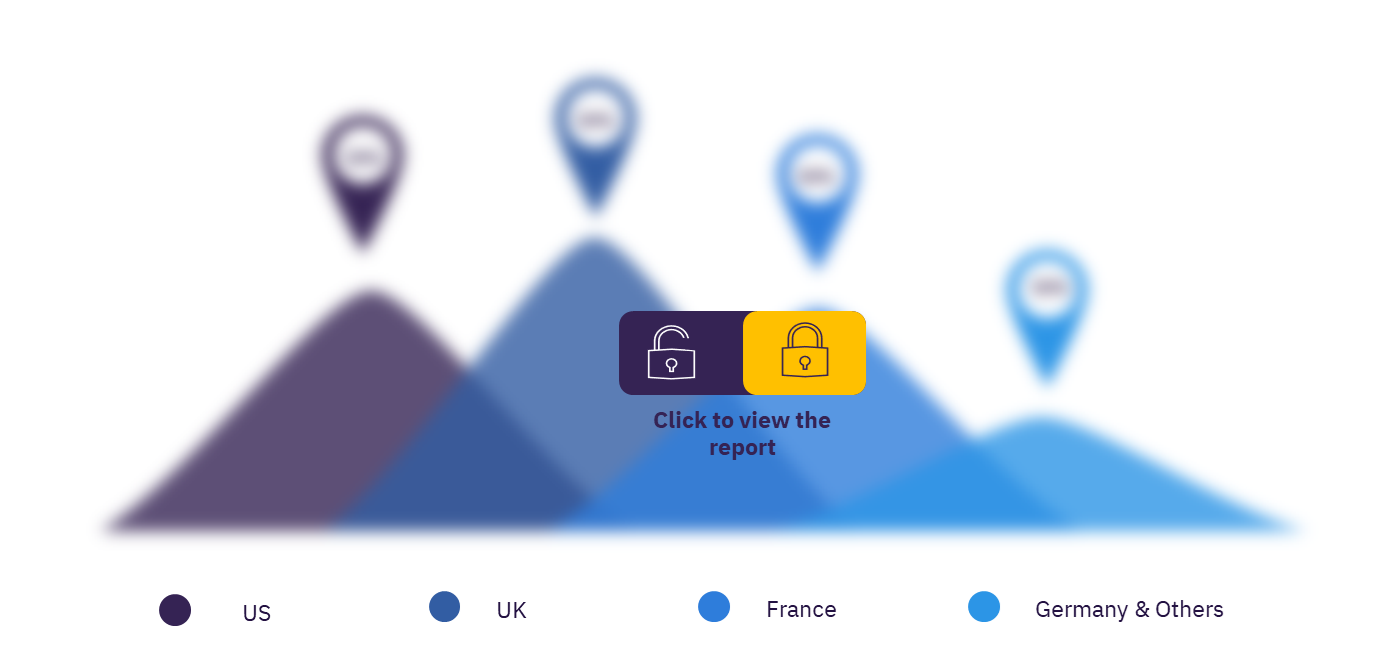
Most of the sales has come from the US, which represents more than 75% of the market by 2030. The US is expected to dominate the cystic fibrosis market throughout the forecast period, as it has the largest cystic fibrosis population with a CAGR of 3.0% over the 10-year timeframe. Following the US, the UK is estimated to have the second highest sales in 2030 owing to high diagnosed prevalent cases. Steady growth across other European countries will be observed over the forecast period.
There are seven major pharmaceutical markets (7MM) covered in this report – The US, 5EU (France, Germany, Italy, Spain, and the UK), and Canada, during the forecast period from 2020-2030.
Overview of the cystic fibrosis market
For more insights on this report, download a free report sample
What are the market dynamics and market barriers for the cystic fibrosis market?
The major drivers of the growth within the cystic fibrosis (CF) market includes: the continued uptake of high-priced Trikafta/Kaftrio and label expansion to a growing population of CF patients, the launch of VX-121 + tezacaftor + VX-561 (deutivacaftor) in 2025 in the US and in 2026 in the 5EU, a high-priced next-generation triple combination, the launch of VX-561 (deutivacaftor) in 2025 in the US and in 2026 in the 5EU, a once-daily CFTR potentiator, which is expected to be more effective than Kalydeco (ivacaftor) and continued growth of the CF patient population due to overall population growth across the 7MM and increasing life expectancy of CF patients.
Also, with this the major barriers to the growth of the market include, the slow reimbursement for novel CFTR modulators in some 5EU countries and Canada, decreased usage of symptomatic drug classes such antibiotics, mucolytics, and anti-inflammatory drugs due to overall improvements to lung health following use of Trikafta/Kaftrio, lack of novel products entering the CF market in the mucolytic or inhaled drug class and low patient compliance due to the high treatment burden.
In 2020, the UK had the highest diagnosed prevalence of cystic fibrosis while Spain had the lowest diagnosed prevalence. Prior to 2020, all markets experienced increases of varying degrees in diagnosed prevalence. After 2020, the CF prevalence continued to increase slightly in France, Germany, and the US while plateauing in the remaining markets.
Registry-based diagnosed prevalent cases of cystic fibrosis
The GlobalData forecast suggests that the diagnosed prevalent cases of CF in 7MM will increase from 69,134 cases in 2020 to 72,659 cases in 2030. In 7MM, the US had the highest number of diagnosed prevalent cases of CF with 32,012 cases, while Spain had the lowest, with 2,281 cases. The changes in the number of diagnosed prevalent cases can therefore be attributed to changes in both the prevalence of CF and the underlying population structure of each market. Furthermore, the US had the highest Annual Growth Rate while Spain had the lowest CAGR growth rate.
Registry-based diagnosed prevalent cases of cystic fibrosis, 7MM, men & women, 2020
For more insights, download a free report sample
How has the global COVID-19 pandemic impacted cystic fibrosis?
The global COVID-19 pandemic has burdened the healthcare systems and disrupted routine medical care for many people. The people with CF suffer from additional comorbid conditions, such as CFRD, that may also put the patients at higher risk for more severe COVID-19-related outcomes. The outcomes found that hospitalization was more likely among older CF patients and those who had CFRD, lower lung function in the year prior to infection, or who received an organ transplant.
People with CF, particularly those who have advanced lung disease or have had a transplant, remained vigilant to reduce exposure to COVID-19. Still, CF researchers indicate that patients infected with COVID-19 have been doing better as compared to COVID-19-related outcomes in other chronic disease groups, possibly due to the relatively younger age of the CF population, lower prevalence of obesity, or other, currently unknown factors.
What is the disease management and treatment for cystic fibrosis?
Diagnosis:
Early Diagnosis: The diagnosis of CF typically begins with an NBS which is the optimal method to diagnose CF during the asymptomatic period. The test uses a few drops of blood from a heel prick to check the levels of immunoreactive trypsinogen (IRT), an enzyme precursor made in the pancreas. If the newborn has a positive NBS result, additional tests such as a sweat test and genetic test will be performed to confirm the diagnosis.
Late Diagnosis:
Since NBS was introduced in the late 2000s, the majority of CF cases were being diagnosed shortly after birth, but sometimes the condition may not be diagnosed until later in life. These rare patients diagnosed in adulthood typically have a mutation associated with residual CFTR function. These individuals present with mild childhood respiratory symptoms but may develop bronchiectasis, pancreatitis, or present with infertility during adulthood. Usually, a sweat test and genetic test confirm the diagnosis of CF in adult patients presenting with CF-like symptoms.
Carrier testing for CF
CF carriers have no symptoms but can pass on the non-functioning gene to their child. Therefore, genetic testing of carriers can identify whether an individual carries a mutation of the CFTR gene.
Treatment guidelines and leading prescribed drugs
When treating CF patients, physicians in the US typically follow guidelines produced by the CFF, physicians in the 5EU (France, Germany, Italy, Spain, and the UK) follow guidelines produced by the European Cystic Fibrosis Society (ECFS), while physicians in Canada follow the Cystic Fibrosis Canada Guidelines.
Some of the leading drugs used in clinical practice to treat CF are Inhaled Antibiotics (aztreonam, colistimethate sodium, levofloxacin, and others), Mucolytics (dornase alfa, mannitol), Pancreatic Enzyme Replacement Therapy (pancrelipase), and CFTR Modulators (ivacaftor, elexacaftor, ezacaftor + ivacaftor and ivacaftor and others).
Which are the key players in the cystic fibrosis market?
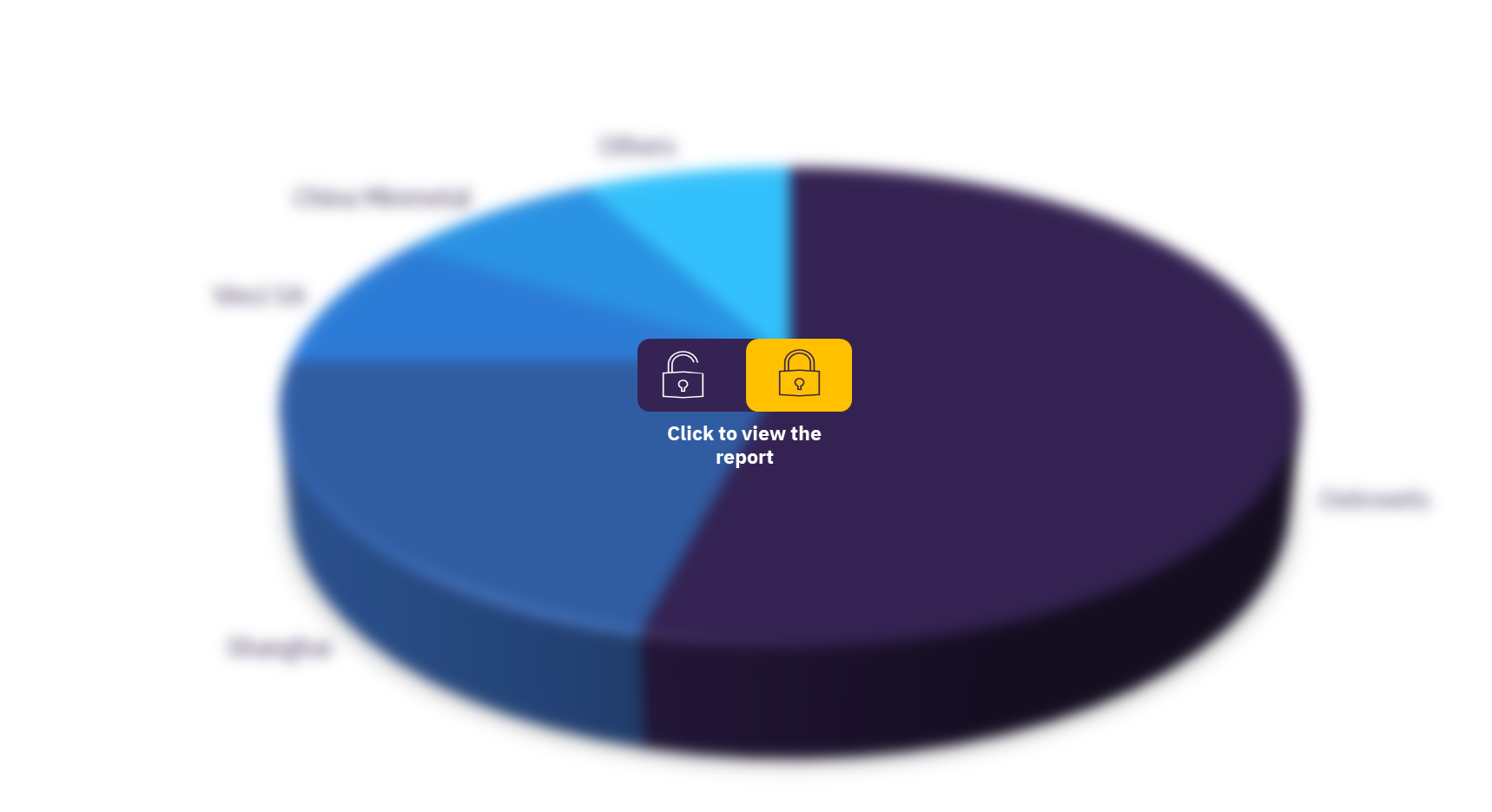
There are over a dozen pharmaceutical companies that are current players in the CF market. Vertex is found to be the market leader, dominating the CF space. While Vertex may currently own the CFTR modulator drug class, other players stand out with marketed products in other drug classes. Vertex and AbbVie are the only companies with stakes in both the current and future CF markets.
Chiesi possesses two inhaled antibiotic products, Bramitob/Bethkis (US and 5EU) and Quinsair (5EU); a mucolytic, Bronchitol (US); and a PERT, Pertzye (US). Additionally, Viatris owns the most widely used inhaled antibiotic therapies, TOBI and TOBI Podhaler, and Nestlé HealthScience owns two PERTs, Zenpep and Viokace.
The rest of the pharmaceutical companies with marketed drugs used for the treatment of CF include Gilead, Teva, Roche/Genentech, AbbVie/Allergan, Vivus, Digestive Care, Horizon Therapeutics, and Pharmaxis. Thus, Laurent Pharmaceuticals is expected to be the only new player in the CF space during the forecast period.
Cystic fibrosis market, by key players
For more insights, download a free report sample
Market report scope
| Market size
(Year – 2020) |
$8.2 billion |
| Growth rate | CAGR of >4% from 2021 to 2025 |
| Base year for estimation | 2020 |
| Market segments by country | The US, France, Germany, Italy, Spain, the UK, and Canada |
| Forecast period | 2021–2025 |
| Key players | Vertex, Chiesi, Viatris, Nestlé HealthScience, Gilead, Teva, Roche/Genentech, AbbVie/Allergan, Vivus, Digestive Care, Horizon Therapeutics, Laurent Pharmaceuticals and Pharmaxis. |
Scope
- Overview of CF including epidemiology, etiology, pathophysiology, symptoms, diagnosis, and treatment guidelines.
- Topline CF market revenue, the annual cost of therapy, and major pipeline product sales in the forecast period.
- Key topics covered include current treatment and pipeline therapies, unmet needs and opportunities, and the drivers and barriers affecting CF therapeutics sales in the 7MM.
- Pipeline analysis: Comprehensive data split across different phases, emerging novel trends under development, and detailed analysis of late-stage pipeline drugs.
- Analysis of the current and future market competition in the global CF therapeutics market. Insightful review of the key industry drivers, restraints, and challenges. Each trend is independently researched to provide a qualitative analysis of its implications.
Key Highlights
The greatest drivers of growth in the global CF market include:
The continued uptake of high-priced Trikafta/Kaftrio and label expansion to a growing population of CF patients
The launch of VX-121 + tezacaftor + VX-561 (deutivacaftor) in 2025 in the US and in 2026 in the 5EU, a high-priced next-generation triple combination.
Continued growth of the CF patient population due to overall population growth across the 7MM and increasing life expectancy of CF patients.
The main barrier to growth in the CF market include:
Slow reimbursement for novel CFTR modulators in some 5EU countries and Canada.
Decreased usage of symptomatic drug classes such antibiotics, mucolytics, and anti-inflammatory drugs due to overall improvements to lung health following use of Trikafta/Kaftrio.
Lack of novel products entering the CF market in the mucolytic or inhaled drug class.
Key unmet needs include the lack of curative therapies, the need for better antibiotic regimens to fight lung infections, limited choice in mucolytic products, and low patient adherence to treatment.
Reasons to Buy
- Develop and design your in-licensing and out-licensing strategies, using a detailed overview of current pipeline products and technologies to identify companies with the most robust pipelines.
- Develop business strategies by understanding the trends shaping and driving the global CF therapeutics market.
- Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the global CF market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counterstrategies to gain a competitive advantage.
- Track drug sales in the global CF therapeutics market from 2020-2030.
- Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments, and strategic partnerships.
Chiesi
Genentech (Roche)
Gilead
Horizon Therapeutics
Laurent Pharmaceuticals
Nestle HealthScience
Pharmaxis
Teva
Vertex Pharmaceuticals
Viatris (formerly Mylan)
Vivus Pharmaceuticals
Table of Contents
Table
Figures
Frequently asked questions
-
What is the value of the cystic fibrosis market in 2020?
The cystic fibrosis market was valued at $8.2 billion in 2020.
-
What are the key countries in the cystic fibrosis market?
There are seven major pharmaceutical markets (7MM) covered in this report: the US, 5EU (France, Germany, Italy, Spain, and the UK), and Canada.
-
Who are the key market players in the cystic fibrosis market?
Vertex is the leading player followed by Chiesi, Viatris, Nestlé HealthScience, Gilead, Teva, Roche/Genentech and, AbbVie/Allergan.
-
What is the growth rate of the cystic fibrosis market?
The cystic fibrosis market is projected to grow at a CAGR of more than 4% during the period 2021-2025.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Respiratory reports