Electrophysiology Devices – Pipeline Products by Stage of Development 21
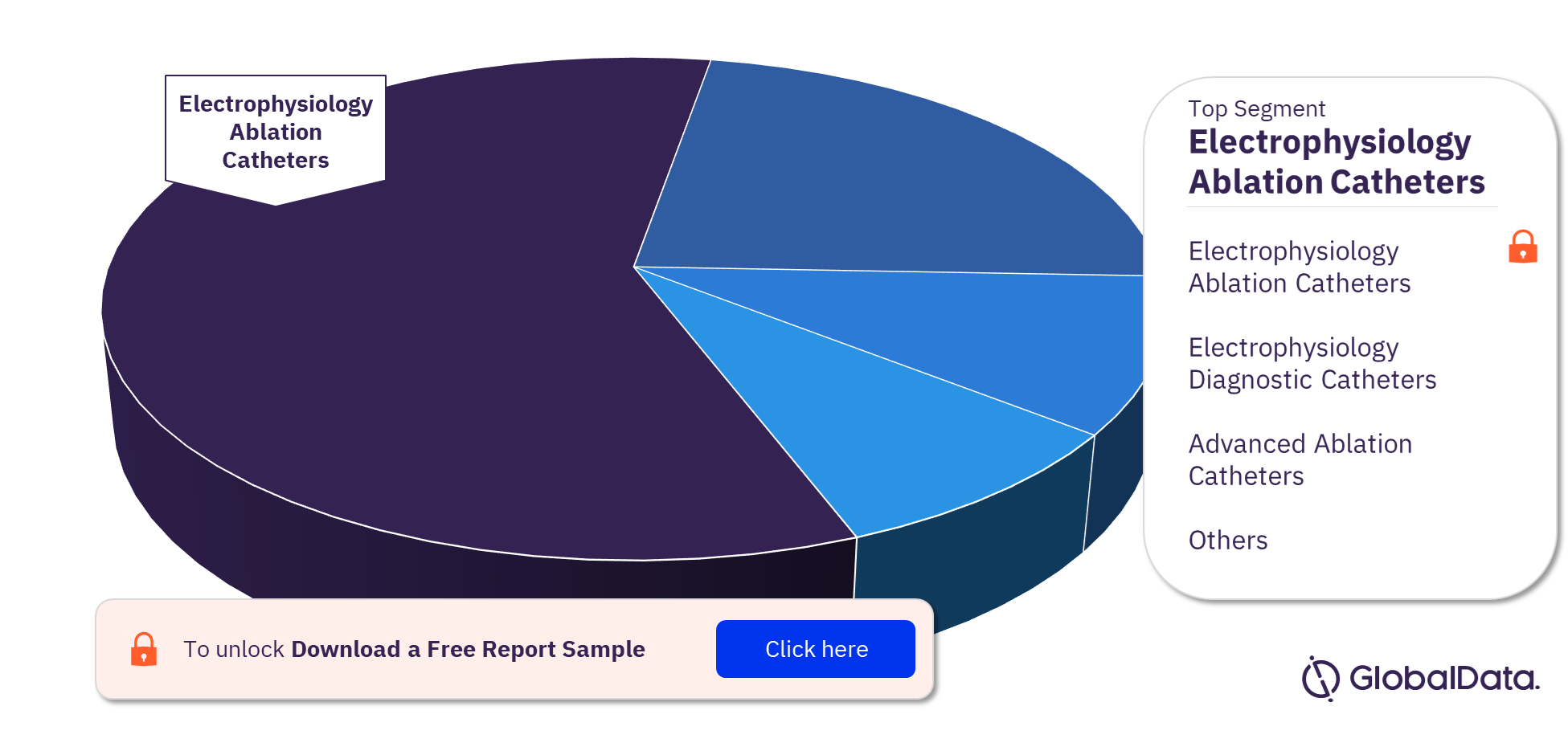
Electrophysiology Devices – Pipeline Products by Segment 22
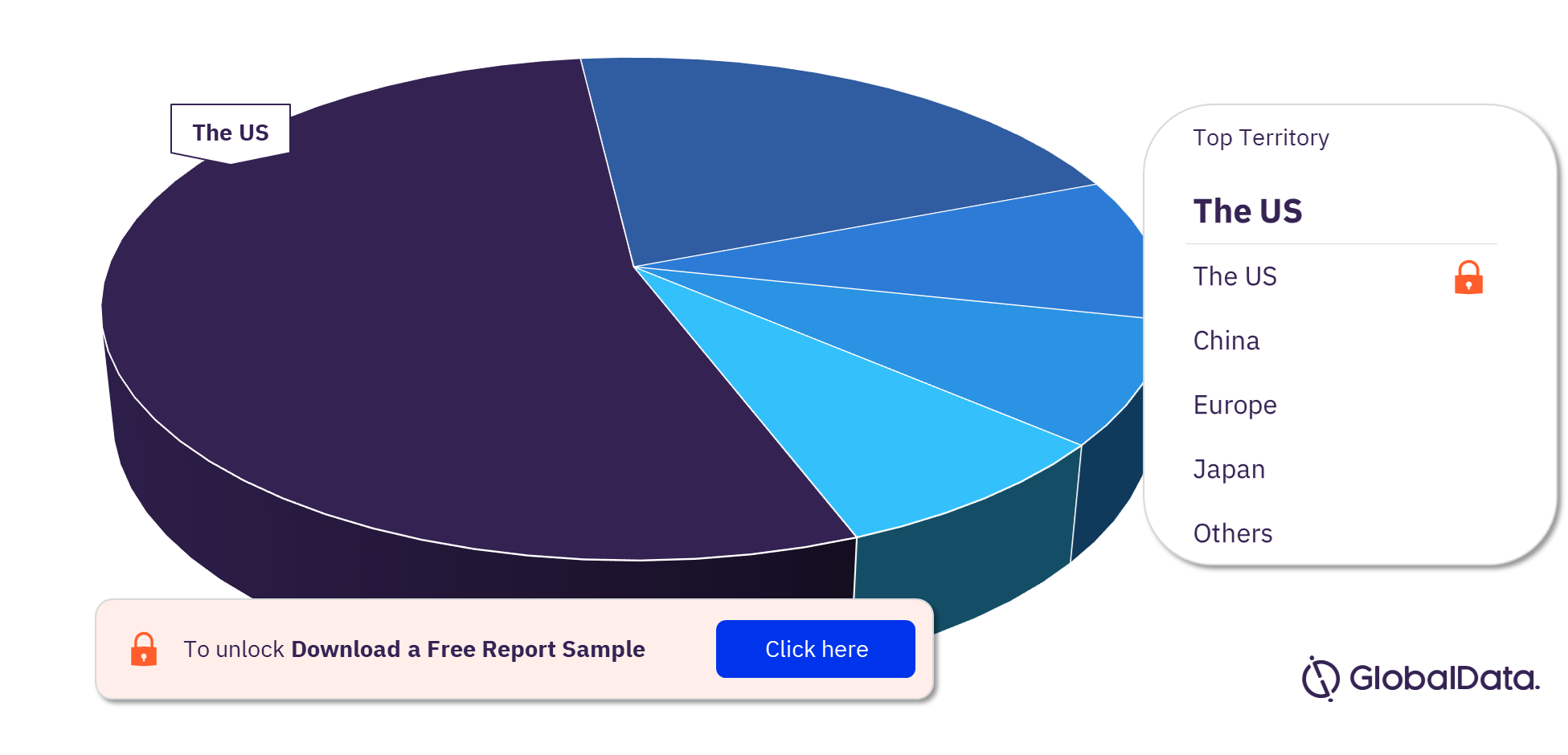
Electrophysiology Devices – Pipeline Products by Territory 23
Electrophysiology Devices – Pipeline Products by Regulatory Path 24
Electrophysiology Devices – Pipeline Products by Estimated Approval Date 25
Electrophysiology Devices – Ongoing Clinical Trials 26
Electrophysiology Devices Companies – Pipeline Products by Stage of Development 27
Electrophysiology Devices – Pipeline Products by Stage of Development 31
Ablacon Inc Pipeline Products & Ongoing Clinical Trials Overview 36
Ablamap System – Product Status 36
Ablamap System – Product Description 36
Ablasense – Product Status 37
Ablasense – Product Description 37
Ablacon Inc – Ongoing Clinical Trials Overview 38
Ablamap System – A Randomized Controlled Study to Evaluate the Reliability of the Ablacon Electrographic FLOW (EGF) Algorithm Technology (Ablamap Software) to Identify AF Sources and Guide Ablation Therapy in Patients with Persistent Atrial Fibrillation 39
AccuPulse Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 40
3D Cardiac Electroanatomic Mapping System – Product Status 40
3D Cardiac Electroanatomic Mapping System – Product Description 40
Acotec Scientific Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 41
ERA-C30 Endovenous Radiofrequency Closure Catheter – Product Status 41
ERA-C30 Endovenous Radiofrequency Closure Catheter – Product Description 41
ERA-C70 Endovenous Radiofrequency Closure Catheter – Product Status 42
ERA-C70 Endovenous Radiofrequency Closure Catheter – Product Description 42
ERA-G5 Endovenous Radiofrequency Closure Generator – Product Status 42
ERA-G5 Endovenous Radiofrequency Closure Generator – Product Description 43
Acutus Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 44
AcQBlate Force Sensing Ablation System – Product Status 44
AcQBlate Force Sensing Ablation System – Product Description 45
AcQMap 3.0 Contact Mapping Catheter – Product Status 45
AcQMap 3.0 Contact Mapping Catheter – Product Description 45
VT Mapping Catheter – Product Status 46
VT Mapping Catheter – Product Description 46
Acutus Medical Inc – Ongoing Clinical Trials Overview 47
AcQBlate Force Sensing Ablation System – Acqblate Force Sensing Ablation System EU Study for Atrial Fibrillation (AcQForce AF-EU) 48
AcQBlate Force Sensing Ablation System – AcQBlate Force Sensing Ablation System US IDE Study for Atrial Fibrillation (AcQForce AF) 48
Apama Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 49
LUMINIZE Radiofrequency (RF) Balloon Catheter – Product Status 49
LUMINIZE Radiofrequency (RF) Balloon Catheter – Product Description 49
APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 50
3D Cardiac Electrophysiological Mapping System – Product Status 50
3D Cardiac Electrophysiological Mapping System – Product Description 51
High Integration Electrophysiological Recording System – Product Status 51
High Integration Electrophysiological Recording System – Product Description 51
Magnetic Positioning Pressure Sensing Radiofrequency Ablation Catheter – Product Status 52
Magnetic Positioning Pressure Sensing Radiofrequency Ablation Catheter – Product Description 52
Magnetoelectric Positioning High-density Mapping Catheter – Product Status 52
Magnetoelectric Positioning High-density Mapping Catheter – Product Description 52
Magnetoelectric Positioning Ring Mapping Catheter – Product Status 53
Magnetoelectric Positioning Ring Mapping Catheter – Product Description 53
Pressure Sensing Ablation Catheter – Product Status 53
Pressure Sensing Ablation Catheter – Product Description 54
Pressure Sensing Pulse Cancellation Fusion Catheter – Product Status 54
Pressure Sensing Pulse Cancellation Fusion Catheter – Product Description 54
RF Ablation Device with Pressure Sensing – Product Status 55
RF Ablation Device with Pressure Sensing – Product Description 55
AtriCure Inc Pipeline Products & Ongoing Clinical Trials Overview 56
Next Generation RF Generator – Product Status 56
Next Generation RF Generator – Product Description 56
Aust Development, LLC Pipeline Products & Ongoing Clinical Trials Overview 57
Endovascular Cardiac Optical Mapping Device – Product Status 57
Endovascular Cardiac Optical Mapping Device – Product Description 57
Baylor College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 58
Catheter-Based Electrophysiological Imaging System – Product Status 58
Catheter-Based Electrophysiological Imaging System – Product Description 58
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 59
Excitation Wave Cancellation Device – Product Status 59
Excitation Wave Cancellation Device – Product Description 59
Berlin Heals GmbH Pipeline Products & Ongoing Clinical Trials Overview 60
C-MIC Device – Product Status 60
C-MIC Device – Product Description 60
Berlin Heals GmbH – Ongoing Clinical Trials Overview 61
C-MIC Device – Early Feasibility Study of C-MIC Heart Failure Device 62
C-MIC Device – Prospective, Randomized, Open, Comparison Study to Demonstrate the Performance and the Safety of Cardiac Microcurrent Therapy (C-MIC) System 62
C-MIC Device – The C-MIC-II Follow-Up Study 62
Biosense Webster Inc Pipeline Products & Ongoing Clinical Trials Overview 63
CARTO 4 – Product Status 63
CARTO 4 – Product Description 63
CARTO SMARTTOUCH 5D Catheter – Product Status 64
CARTO SMARTTOUCH 5D Catheter – Product Description 64
Heliostar Multi-Electrode Radiofrequency Balloon Ablation Catheter – Product Status 64
Heliostar Multi-Electrode Radiofrequency Balloon Ablation Catheter – Product Description 65
Navigation Guided Deflectable Sheath – Product Status 65
Navigation Guided Deflectable Sheath – Product Description 65
THERMOCOOL SMARTTOUCH SF-5D Catheter – Product Status 66
THERMOCOOL SMARTTOUCH SF-5D Catheter – Product Description 66
TRUPULSE Generator – Product Status 66
TRUPULSE Generator – Product Description 67
Biosense Webster Inc – Ongoing Clinical Trials Overview 68
Heliostar Multi-Electrode Radiofrequency Balloon Ablation Catheter – Implementation of the HELIOSTAR in Real-world Clinical Practice at a High-volume Center – Operator Learning Curve and Procedural Outcome Parameters 69
CARTO SMARTTOUCH 5D Catheter – Ground-Breaking Electroporation-based Intervention for PERSistent Atrial Fibrillation Treatment (BEAT PERS-AF) 70
THERMOCOOL SMARTTOUCH SF-5D Catheter – Evaluation of the Safety and Performance of the Very High Power-short Duration QDOT Strategy in Patients Referred for Atrial Fibrillation Ablation 71
TRUPULSE Generator – Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation With the BWI IRE Ablation System (AdmIRE) 72
TRUPULSE Generator – Pulsed Field Ablation (PFA) System for the Treatment of Paroxysmal Atrial Fibrillation (PAF) by Irreversible Electroporation (IRE) 72
TRUPULSE Generator – Safety and Effectiveness Evaluation of the THERMOCOOL SMARTTOUCH SF Catheter With the TRUPULSE Generator for Treatment of Paroxysmal Atrial Fibrillation (PAF) 72
BioSig Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 73
PURE EP System – Product Status 73
PURE EP System – Product Description 74
PURE EP Version 2.0 – Product Status 74
PURE EP Version 2.0 – Product Description 74
PURE EP Version 3.0 – Product Status 75
PURE EP Version 3.0 – Product Description 75
BioSig Technologies Inc – Ongoing Clinical Trials Overview 76
PURE EP System – To Assess the Efficacy Rate of Unipolar Polarity Switch for Lesion Assessment in Pulmonary Vein Isolation 77
PURE EP System – To Assess the Predictive Value of High Frequency Algorithm of PURE EP for Low Amplitude Signal Detection 77
PURE EP System – Validation of Safety and efficacy of the PURE EP System During Mapping and Ablation Procedures in Cardiac EP lab 77
BioTex Inc Pipeline Products & Ongoing Clinical Trials Overview 78
Cooled Tip Catheter – Product Status 78
Cooled Tip Catheter – Product Description 78
Biotronik AG Pipeline Products & Ongoing Clinical Trials Overview 79
Amvia Edge – Product Status 79
Amvia Edge – Product Description 79
Amvia Sky – Product Status 80
Amvia Sky – Product Description 80
Truvia – Product Status 80
Truvia – Product Description 80
Biotronik AG – Ongoing Clinical Trials Overview 81
Amvia Sky – BIO|CONCEPT.Amvia, First in Human Study for the Amvia/Solvia Pacemaker Family 82
Biotronik Australia Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 83
ElePulse System – Product Status 83
ElePulse System – Product Description 83
Biotronik Australia Pty Ltd – Ongoing Clinical Trials Overview 84
ElePulse System – Rapid One-Shot Electroporation Trial For Atrial Fibrillation: Reset-AF: Investigating the Effect of A Novel Intervention on Atrial Fibrillation Recurrence 85
Biotronik SE & Co KG Pipeline Products & Ongoing Clinical Trials Overview 86
SentiCath – Product Status 86
SentiCath – Product Description 86
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 87
Intellagen – Product Status 87
Intellagen – Product Description 88
Intellanav Stablepoint – Product Status 88
Intellanav Stablepoint – Product Description 88
LabSystem Pro NextGen – Product Status 89
LabSystem Pro NextGen – Product Description 89
Lesion Assessment Device – Product Status 89
Lesion Assessment Device – Product Description 89
Next Generation Blazer Catheter – Product Status 90
Next Generation Blazer Catheter – Product Description 90
Next Generation IntellaTip Catheter – Product Status 90
Next Generation IntellaTip Catheter – Product Description 91
OI System – Product Status 91
OI System – Product Description 91
POLARx Next Generation Catheter – Product Status 92
POLARx Next Generation Catheter – Product Description 92
POLARx Single Shot Cryoballoon – Product Status 92
POLARx Single Shot Cryoballoon – Product Description 93
Rhythmia 5.0 – Product Status 93
Rhythmia 5.0 – Product Description 93
Boston Scientific Corp – Ongoing Clinical Trials Overview 94
Intellanav Stablepoint – Clinical Evaluation of the StablePoint Catheter and Force Sensing System for Paroxysmal Atrial Fibrillation 95
POLARx Single Shot Cryoballoon – Comparison of the PolarX and the Arctic Front Cryoballoon for Pulmonary Vein Isolation in Patients with Symptomatic Paroxysmal Atrial Fibrillation – A Multi-center Non-inferiority Design Clinical Trial 96
POLARx Single Shot Cryoballoon – Safety and Effectiveness IDE Trial for Boston Scientific’s Cryoballoon in the Treatment of Symptomatic Drug Refractory Paroxysmal Atrial Fibrillation 96
Cardima, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 97
Cadence EPL – Product Status 97
Cadence EPL – Product Description 97
EP Ablation System – Product Status 98
EP Ablation System – Product Description 98
CardioFocus Inc Pipeline Products & Ongoing Clinical Trials Overview 99
HeartLight X3 System – Product Status 99
HeartLight X3 System – Product Description 99
CardioFocus Inc – Ongoing Clinical Trials Overview 100
HeartLight X3 System – A Prospective Multicenter U.S. Comparison of Radiofrequency, Cryoballoon and Laser Balloon Modalities for Pulmonary Vein Isolation Ablation in Patients with Atrial Fibrillation 101
CardioNova Ltd Pipeline Products & Ongoing Clinical Trials Overview 102
Electrophysiology Catheter – Atrial Fibrillation – Product Status 102
Electrophysiology Catheter – Atrial Fibrillation – Product Description 102
CathEffects LLC Pipeline Products & Ongoing Clinical Trials Overview 103
Desai VectorCathRF1 Mapping And Ablation Catheter – Product Status 103
Desai VectorCathRF1 Mapping And Ablation Catheter – Product Description 103
catheter precision Inc Pipeline Products & Ongoing Clinical Trials Overview 104
Second Generation Amigo – Product Status 104
Second Generation Amigo – Product Description 104
CathRx Ltd Pipeline Products & Ongoing Clinical Trials Overview 105
CathRx 4mm Therapeutic Catheter – Product Status 105
CathRx 4mm Therapeutic Catheter – Product Description 105
CathRx 8mm Therapeutic Catheter – Product Status 106
CathRx 8mm Therapeutic Catheter – Product Description 106
Khelix Irrigated Ablation EP Catheter – Product Status 106
Khelix Irrigated Ablation EP Catheter – Product Description 107
Khelix Non-Irrigated Ablation EP Catheter – Product Status 107
Khelix Non-Irrigated Ablation EP Catheter – Product Description 107
CathVision Aps Pipeline Products & Ongoing Clinical Trials Overview 108
CathVision ECGenius System – Product Status 108
CathVision ECGenius System – Product Description 108
CathVision Aps – Ongoing Clinical Trials Overview 109
CathVision ECGenius System – A Prospective Single Center Pilot Study Using the ECGenius System to Collect Electrogram Data to Test an Ablation Impact Algorithm 110
CathVision ECGenius System – A Prospective, Single-Center, Open-Label, Single-Arm Study to Evaluate the Safety and Technical Performance of the CathVision ECGenius System 110
CathVision ECGenius System – The Automated Calculation of AF Cycle Length and Complexity Using a Novel EP Recording System (The CathVision ECGenius System) 110
Cibiem, Inc. Pipeline Products & Ongoing Clinical Trials Overview 111
Catheter-Based Carotid Body Ablation System – Product Status 111
Catheter-Based Carotid Body Ablation System – Product Description 111
ClearPoint Neuro Inc Pipeline Products & Ongoing Clinical Trials Overview 112
ClearTrace Cardiac Intervention System – Product Status 112
ClearTrace Cardiac Intervention System – Product Description 113
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 114
Optically-Integrated RFA Catheter – Product Status 114
Optically-Integrated RFA Catheter – Product Description 114
Conavi Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 115
Foresight Intracardiac Echocardiograph System – Product Status 115
Foresight Intracardiac Echocardiograph System – Product Description 115
Conavi Medical Inc – Ongoing Clinical Trials Overview 116
Foresight Intracardiac Echocardiograph System – Visualization of Human Cardiovascular Anatomy with Foresight Intracardiac Echocardiography (ICE) System 117
Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview 118
EP Contact Force Sensing Catheter Enabled By CARTO 3 – Product Status 118
EP Contact Force Sensing Catheter Enabled By CARTO 3 – Product Description 118
EP Lesion Assessment Device – Product Status 119
EP Lesion Assessment Device – Product Description 119
CoreMap Inc Pipeline Products & Ongoing Clinical Trials Overview 120
Electrophysiology Mapping System – Atrial Fibrillation – Product Status 120
Electrophysiology Mapping System – Atrial Fibrillation – Product Description 120
CoRepair, Inc Pipeline Products & Ongoing Clinical Trials Overview 121
Radiofrequency Device – Product Status 121
Radiofrequency Device – Product Description 121
Corify Care SL Pipeline Products & Ongoing Clinical Trials Overview 122
Acorys – Product Status 122
Acorys – Product Description 122
Corify Care SL – Ongoing Clinical Trials Overview 123
Acorys – Non-invasive Tool to Assess Electrophysiological Mechanisms in Cardiac Arrhythmias (SAVE-COR) 124
CryoTherapeutics Gmbh Pipeline Products & Ongoing Clinical Trials Overview 125
Cryotherapy System – Coronary Artery Disease – Product Status 125
Cryotherapy System – Coronary Artery Disease – Product Description 125
CryoTherapeutics Gmbh – Ongoing Clinical Trials Overview 126
Cryotherapy System – Coronary Artery Disease – Clinical Investigation of Intracoronary Cryotherapy Using the CryoTherapy System (CTS) for High-risk Plaque in Patients with Non-ST Segment Elevation Myocardial Infarction (NSTEMI) or Unstable Angina 127
CyberHeart Inc Pipeline Products & Ongoing Clinical Trials Overview 128
CyberHeart System – Product Status 128
CyberHeart System – Product Description 128
CyberHeart Inc – Ongoing Clinical Trials Overview 129
CyberHeart System – CyberHeart’s Cardiac Arrhythmia Ablation Treatment: Patients with Refractory Ventricular Tachycardia 130
EPD Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 131
KODEX-EPD System – Balloon Visualization – Product Status 131
KODEX-EPD System – Balloon Visualization – Product Description 131
EPD Solutions Ltd – Ongoing Clinical Trials Overview 132
KODEX-EPD System – Balloon Visualization – Prospective Procedural Data Collection for Continuous Improvement of the KODEX – EPD System Performance 133
Epicardial Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 134
Epicardial Access Device – Product Status 134
Epicardial Access Device – Product Description 134
EPMap-System GmbH & Co KG Pipeline Products & Ongoing Clinical Trials Overview 135
EP Map System – Product Status 135
EP Map System – Product Description 136
Ethicon US LLC Pipeline Products & Ongoing Clinical Trials Overview 137
Next Generation Ablation Catheter – Product Status 137
Next Generation Ablation Catheter – Product Description 137
Eximo Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 138
AURYON Atherectomy System – CAD – Product Status 138
AURYON Atherectomy System – CAD – Product Description 138
Farapulse Inc Pipeline Products & Ongoing Clinical Trials Overview 139
FARAFLEX – Product Status 139
FARAFLEX – Product Description 139
FARAONE – Product Status 140
FARAONE – Product Description 140
FARAPOINT – Product Status 140
FARAPOINT – Product Description 141
Next Gen FARAWAVE Catheter – Product Status 141
Next Gen FARAWAVE Catheter – Product Description 141
FocusStart LLC Pipeline Products & Ongoing Clinical Trials Overview 142
Anti-coagulum Cardiac Ablation Catheter – Product Status 142
Anti-coagulum Cardiac Ablation Catheter – Product Description 142
Fundacion para la Investigacion Biomedica del Hospital Gregorio Maranon Pipeline Products & Ongoing Clinical Trials Overview 143
Fibrillation Catheter – Product Status 143
Fibrillation Catheter – Product Description 143
Galvanize Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 144
QuickShot – Product Status 144
QuickShot – Product Description 144
Galvanize Therapeutics Inc – Ongoing Clinical Trials Overview 145
QuickShot – The First Human Use of QuickShot – A Novel Contact-Sensing, Large Area, Focal Pulsed Electric Field Mapping & Ablation Catheter 146
Hansen Medical Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 147
Sensei Robotic Catheter System – Percutaneous Aortic Valve Replacement – Product Status 147
Sensei Robotic Catheter System – Percutaneous Aortic Valve Replacement – Product Description 147
Sensei X Robotic Catheter System – Atrial Fibrillation – Product Status 148
Sensei X Robotic Catheter System – Atrial Fibrillation – Product Description 148
Hyblate Medical Pipeline Products & Ongoing Clinical Trials Overview 149
Hybrid Ablation Catheter – Product Status 149
Hybrid Ablation Catheter – Product Description 149
Imricor Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 150
Advantage-MR EP Recorder/Stimulator System – Product Status 150
Advantage-MR EP Recorder/Stimulator System – Product Description 151
Vision-MR Ablation Catheter – Product Status 151
Vision-MR Ablation Catheter – Product Description 151
Vision-MR Ablation Catheter 2.0 – Product Status 152
Vision-MR Ablation Catheter 2.0 – Product Description 152
Vision-MR Diagnostic Catheter – Product Status 152
Vision-MR Diagnostic Catheter – Product Description 153
Vision-MR Dispersive Electrode – Product Status 153
Vision-MR Dispersive Electrode – Product Description 153
Imricor Medical Systems Inc – Ongoing Clinical Trials Overview 154
Vision-MR Ablation Catheter 2.0 – A Global, Prospective Study of Vision-MR Ablation of VisionMR Ablation Catheter (Vision-MR Ablation of Atrial FLutter) – VISABL-ALF 155
Vision-MR Ablation Catheter 2.0 – Vision-MR Ablation Catheter 2.0 for the Treatment of Ventricular Tachycardia 155
Interventional Imaging, Inc. Pipeline Products & Ongoing Clinical Trials Overview 156
Atrial Fibrillation Catheter – Product Status 156
Atrial Fibrillation Catheter – Product Description 156
Iowa Approach Inc. Pipeline Products & Ongoing Clinical Trials Overview 157
MaPP-CS – Product Status 157
MaPP-CS – Product Description 157
ROF-AC – Product Status 158
ROF-AC – Product Description 158
ROF-AS – Product Status 158
ROF-AS – Product Description 159
Japan Lifeline Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 160
ALPHA1 Ablation Catheter – Product Status 160
ALPHA1 Ablation Catheter – Product Description 161
Jiangsu Tingsheng Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 162
2D Intracardiac Ultrasound – Product Status 162
2D Intracardiac Ultrasound – Product Description 162
4D Intracardiac Ultrasound – Product Status 163
4D Intracardiac Ultrasound – Product Description 163
Disposable Intracardiac Ultrasound Catheter – Product Status 163
Disposable Intracardiac Ultrasound Catheter – Product Description 164
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 165
RF Ablation Lesion Assessment System – Product Status 165
RF Ablation Lesion Assessment System – Product Description 165
King’s College London Pipeline Products & Ongoing Clinical Trials Overview 166
Steerable Catheter – Product Status 166
Steerable Catheter – Product Description 166
Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview 167
FL.ICE Catheter – Product Status 167
FL.ICE Catheter – Product Description 167
Lepu Medical Technology (Beijing) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 168
Pulsed Acoustical Generator – Product Status 168
Pulsed Acoustical Generator – Product Description 168
Lepu Scientech Medical Technology (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 169
Radiofrequency Ablation Device – Product Status 169
Radiofrequency Ablation Device – Product Description 169
Maestro Heart SA Pipeline Products & Ongoing Clinical Trials Overview 170
Maestro-AF Catheter System – Product Status 170
Maestro-AF Catheter System – Product Description 170
MC10 Inc Pipeline Products & Ongoing Clinical Trials Overview 171
Sensor-Covered Cardiac Catheter – Product Status 171
Sensor-Covered Cardiac Catheter – Product Description 171
Medlumics SL Pipeline Products & Ongoing Clinical Trials Overview 172
AblaView – Product Status 172
AblaView – Product Description 172
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 173
Osteocool 2.0 – Product Status 173
Osteocool 2.0 – Product Description 174
Sphere-9 – Product Status 174
Sphere-9 – Product Description 174
Symplicity G3 Generator – Product Status 175
Symplicity G3 Generator – Product Description 175
Medtronic Plc – Ongoing Clinical Trials Overview 176
Symplicity G3 Generator – Cardiac Sympathetic Neuromodulation by Renal Denervation in Hypertrophic Cardiomyopathy (SNYPER Pilot Study) 177
Sphere-9 – Treatment of Persistent Atrial Fibrillation with the Sphere-9 Mapping and Ablation Catheter and the Affera Mapping and Ablation System 178
MedWaves Inc Pipeline Products & Ongoing Clinical Trials Overview 179
Arrhythmia Catheters – Product Status 179
Arrhythmia Catheters – Product Description 179
Medyria AG Pipeline Products & Ongoing Clinical Trials Overview 180
PhysioCath System – Product Status 180
PhysioCath System – Product Description 180
MicroPort NeuroTech Ltd Pipeline Products & Ongoing Clinical Trials Overview 181
Renal RF Ablation System – Product Status 181
Renal RF Ablation System – Product Description 181
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 182
Circular Ablation Catheter – Product Status 182
Circular Ablation Catheter – Product Description 182
Circular Diagnostic Catheter – Product Status 183
Circular Diagnostic Catheter – Product Description 183
Variable Loop – Product Status 183
Variable Loop – Product Description 184
Voyager Irrigated RF Ablation Catheter – Product Status 184
Voyager Irrigated RF Ablation Catheter – Product Description 184
NeuTrace Inc Pipeline Products & Ongoing Clinical Trials Overview 185
AI-Guided Cardiac Mapping Device – Product Status 185
AI-Guided Cardiac Mapping Device – Product Description 185
NeuTrace Inc – Ongoing Clinical Trials Overview 186
AI-Guided Cardiac Mapping Device – Physician Acceptance of the NeuTrace System for Cardiac Electroanatomic Mapping 187
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 188
Cardiac Catheter – Product Status 188
Cardiac Catheter – Product Description 188
Novasentis Inc Pipeline Products & Ongoing Clinical Trials Overview 189
Electroactive Polymer Based Steerable Catheter – Product Status 189
Electroactive Polymer Based Steerable Catheter – Product Description 189
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 190
Ablation Catheter – Product Status 190
Ablation Catheter – Product Description 190
Mapping Catheter – Product Status 191
Mapping Catheter – Product Description 191
Philips Healthcare Pipeline Products & Ongoing Clinical Trials Overview 192
Integrated X-Ray And Robotic Catheter System – Product Status 192
Integrated X-Ray And Robotic Catheter System – Product Description 192
S4 Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 193
esolution Device – Product Status 193
esolution Device – Product Description 193
SCR, Inc. Pipeline Products & Ongoing Clinical Trials Overview 194
Atrial Fibrillation Device – Product Status 194
Atrial Fibrillation Device – Product Description 194
Seattle Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 195
Ablation Catheter – Heart Arrhythmia – Product Status 195
Ablation Catheter – Heart Arrhythmia – Product Description 195
Seoul National University Hospital Pipeline Products & Ongoing Clinical Trials Overview 196
Cardiac Stimulator – Product Status 196
Cardiac Stimulator – Product Description 196
Shanghai HeartCare Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 197
Cryoablation Catheter – Product Status 197
Cryoablation Catheter – Product Description 197
Shanghai MicroPort EP MedTech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 198
Contact Force-Sensing RF Ablation Catheter – Product Status 198
Contact Force-Sensing RF Ablation Catheter – Product Description 199
EasyFinder 3D Star High Density Mapping Catheter – Product Status 199
EasyFinder 3D Star High Density Mapping Catheter – Product Description 199
EasyLoop Intracardiac Mapping Catheter – Product Status 200
EasyLoop Intracardiac Mapping Catheter – Product Description 200
FireMagic TT 3D Ablation Catheter – Product Status 200
FireMagic TT 3D Ablation Catheter – Product Description 200
IceMagic Cryoablation Catheter – Product Status 201
IceMagic Cryoablation Catheter – Product Description 201
IceMagic Cryoablation System – Product Status 201
IceMagic Cryoablation System – Product Description 202
ST Cardio Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 203
Z6 Cardiac Stimulator With Integrated iPad – Product Status 203
Z6 Cardiac Stimulator With Integrated iPad – Product Description 203
St. Jude Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 204
All-in-one Catheter Ablation System – Product Status 204
All-in-one Catheter Ablation System – Product Description 204
FlexAbility Irrigated Ablation Catheter Sensor Enabled – Monomorphic Ventricular Tachycardia – Product Status 205
FlexAbility Irrigated Ablation Catheter Sensor Enabled – Monomorphic Ventricular Tachycardia – Product Description 205
St. Jude Medical LLC – Ongoing Clinical Trials Overview 206
FlexAbility Irrigated Ablation Catheter Sensor Enabled – Monomorphic Ventricular Tachycardia – Flexability Sensor Enabled Substrate Targeted Ablation for the Reduction of Ventricular Tachycardia (LESS-VT) Study 207
Stanford University Pipeline Products & Ongoing Clinical Trials Overview 208
Cardiac-Mapping Catheter – Product Status 208
Cardiac-Mapping Catheter – Product Description 208
Stereotaxis Inc Pipeline Products & Ongoing Clinical Trials Overview 209
MAGiC Ablation Catheter – Product Status 209
MAGiC Ablation Catheter – Product Description 209
Next Generation Irrigated Magnetic Catheter – Product Status 210
Next Generation Irrigated Magnetic Catheter – Product Description 210
Robotic Magnetic Navigation System – Pulmonary Hypertension – Product Status 210
Robotic Magnetic Navigation System – Pulmonary Hypertension – Product Description 211
Stereotaxis Genesis RMN – Product Status 211
Stereotaxis Genesis RMN – Product Description 211
Suzhou Aikemai Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 212
3D Mapping System – Product Status 212
3D Mapping System – Product Description 212
Suzhou Bingjing Intelligent Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 213
3D Imaging ICE Device – Product Status 213
3D Imaging ICE Device – Product Description 213
4D Imaging ICE Device – Product Status 214
4D Imaging ICE Device – Product Description 214
HD-ICE – Product Status 214
HD-ICE – Product Description 215
Suzhou Hengruihongyuan Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 216
Endovenous Radiofrequency Ablation Closure System – Product Status 216
Endovenous Radiofrequency Ablation Closure System – Product Description 216
Suzhou Hengruihongyuan Medical Technology Co Ltd – Ongoing Clinical Trials Overview 217
Endovenous Radiofrequency Ablation Closure System – Prospective, Multi-center, Randomized Controlled Study to Evaluate the Effectiveness and Safety of Endovenous Radiofrequency Ablation Closure System in the Treatment of Varicose Veins 218
Suzhou Xinmai Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 219
RF System – Product Status 219
RF System – Product Description 219
The Cleveland Clinic Foundation Pipeline Products & Ongoing Clinical Trials Overview 220
EP Mapping Catheter – Product Status 220
EP Mapping Catheter – Product Description 220
University of Arkansas at Little Rock Pipeline Products & Ongoing Clinical Trials Overview 221
Robotic Catheter – Product Status 221
Robotic Catheter – Product Description 221
University of Auckland Pipeline Products & Ongoing Clinical Trials Overview 222
3D Heart Mapping Device – Product Status 222
3D Heart Mapping Device – Product Description 222
University of Maryland Pipeline Products & Ongoing Clinical Trials Overview 223
Steerable Actuated Catheter – Product Status 223
Steerable Actuated Catheter – Product Description 223
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 224
Non-Contact Mapping Catheter – Product Status 224
Non-Contact Mapping Catheter – Product Description 224
University of Rochester Pipeline Products & Ongoing Clinical Trials Overview 225
RF Ablation Catheter – Product Status 225
RF Ablation Catheter – Product Description 225
University of Texas Medical Branch at Galveston Pipeline Products & Ongoing Clinical Trials Overview 226
Ablation Catheter – Product Status 226
Ablation Catheter – Product Description 226
University of Utah Pipeline Products & Ongoing Clinical Trials Overview 227
Cardiac Tissue Imaging Catheter – Product Status 227
Cardiac Tissue Imaging Catheter – Product Description 227
Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 228
Temperature Controlled Catheter – Product Status 228
Temperature Controlled Catheter – Product Description 228
Vimecon GmbH Pipeline Products & Ongoing Clinical Trials Overview 229
Vimecon Laser CAI Catheter – Product Status 229
Vimecon Laser CAI Catheter – Product Description 229
Viscardia Inc Pipeline Products & Ongoing Clinical Trials Overview 230
VisONE System – Product Status 230
VisONE System – Product Description 230
Viscardia Inc – Ongoing Clinical Trials Overview 231
VisONE System – RECOVER-HF Pilot Study – Randomized, Single-Center, Double-blinded Study of Synchronized Diaphragmatic Stimulation (SDS) for Improvement of Symptomatic Reduced Ejection Fraction Heart Failure 232
Vivonics Inc Pipeline Products & Ongoing Clinical Trials Overview 233
Catheter Guidance System – Product Status 233
Catheter Guidance System – Product Description 233
Voyage Medical (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 234
IRIS Ablation System – Product Status 234
IRIS Ablation System – Product Description 234
Zylox-Tonbridge Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 235
Radiofrequency Generator – Product Status 235
Radiofrequency Generator – Product Description 235
Glossary 277
![]()