Coronavirus Disease 2019 (COVID-19) Analyst Consensus Sales Analysis and Forecast, H2 2022
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
COVID-19 Analyst Consensus Sales Report Overview
Prophylactic COVID-19 vaccines are projected to generate total sales of $264 billion from 2021 to 2028. The number of COVID-19 drugs with forecast sales nearly tripled due to the massive mandatory vaccination campaigns led by the major markets, which caused rapid increases in COVID-19 vaccines. BioNTech/Pfizer’s Comirnaty, Moderna’s Spikevax, and Pfizer’s Paxlovid are the top three marketed drugs with the highest peak forecast sales. Comparing Q4 2020 and H2 2022 total COVID-19 forecast sales within the 5-year period of 2021-26, forecast sales have grown considerably, with the H2 2022 forecast being greater than Q4 2020 forecasts.
The COVID-19 analyst consensus sales market research report contains a summary of the analyst consensus forecasts available in the GlobalData pharma intelligence center drug sales and analyst consensus database for the indication COVID-19.
COVID-19 Analyst Consensus Sales Forecast by Prophylactic Vaccines
Some of the key prophylactic vaccines analyzed for COVID-19 analyst consensus sales are Comirnaty, Spikevax, Nuvaxovid, Vaxzevria, COVID-19 Vaccine, VidPrevtyn Beta, VLA-2001, Convidecia, Covaxin, and Coronavirus Disease 2019 (COVID-19) vaccine. Comirnaty continues to be the leading prophylactic vaccine by forecast sales. Both Spikevax and Comirnaty have gained approval for their bivalent vaccines.
For more insights on COVID-19 prophylactic drugs analyst consensus sales forecast, download a free report sample
COVID-19 Analyst Consensus Sales Forecast by Therapeutic Drugs
Some of the key therapeutic drugs analyzed for COVID-19 analyst consensus sales are Paxlovid, Veklury, Regen-Cov, Lagevrio, Evusheld, Bamlanivimab/Etesevimab, Xevudy, Bebtelovimab, Amubarvimab + Romlusevimab, and Regkirona. Paxlovid is currently the leading therapeutic drug by forecast sales. Paxlovid and Lagevrio are the only two approved oral antiviral COVID-19 therapeutics on the market.
For more insights on COVID-19 therapeutic drugs analyst consensus sales forecast, download a free report sample
COVID-19 Analyst Consensus Sales Forecast by Drugs
The top three marketed drugs with the highest peak forecast sales are Pfizer/BioNTech SE’s Comirnaty, Moderna’s Spikevax, and Pfizer’s Paxlovid. The top three forecast drugs are all currently marketed, suggesting that the COVID-19 market has matured and that it will be hard for new products to supplant these leading drugs. This report provides an overview of the sales forecast for 52 drugs in clinical development or marketed for COVID-19.
COVID-19 Global Analyst Consensus Sales Forecast Analysis, by Drugs
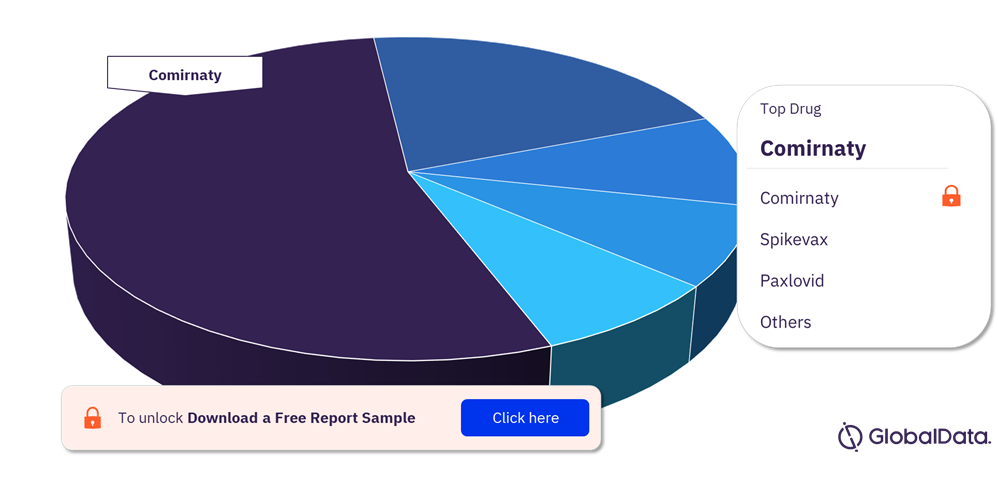 For more drug insights into the COVID-19 analyst consensus sales forecast, download a free report sample
For more drug insights into the COVID-19 analyst consensus sales forecast, download a free report sample
COVID-19 Analyst Consensus Sales Forecast - Top Three Marketed COVID-19 Drugs
The top three marketed COVID-19 forecast drugs include two prophylactic vaccines – Comirnaty and Spikevax – and one therapeutic drug – Paxlovid. The forecast sales of both Comirnaty and Spikevax are expected to decline from 2022 onwards, despite novel bivalent vaccine formulations, due to a reduction in mass vaccination programs. The forecast sales of Spikevax are expected to peak in 2022. The sales of Comirnaty already peaked in 2021. Furthermore, the forecast sales of Paxlovid are expected to peak in 2022 due to its approval at the end of 2021.
To know more about top marketed COVID-19 drugs with peak forecast, download a free report sample
COVID-19 Analyst Consensus Sales Report Overview
| Key Prophylactic Vaccines | Comirnaty, Spikevax, Nuvaxovid, Vaxzevria, COVID-19 Vaccine, VidPrevtyn Beta, VLA-2001, Convidecia, Covaxin, and Coronavirus Disease 2019 (COVID-19) vaccine |
| Key Therapeutic Drugs | Paxlovid, Veklury, Regen-Cov, Lagevrio, Evusheld, Bamlanivimab/Etesevimab, Xevudy, Bebtelovimab, Amubarvimab + Romlusevimab, and Regkirona |
Reasons to Buy
- Analysis of COVID-19 analyst consensus sales forecasts
- Detailed Analysis of how COVID-19 have affected analyst consensus sales forecasts for previously forecasted drugs
- Detailed analysis of how COVID-19 forecasts changed from 2020 to 2022 and what drove these changes
Moderna Inc
Regeneron Pharmaceuticals Inc
Gilead Sciences Inc
AstraZeneca Plc
Johnson & Johnson
Eli Lilly and Co
Vir Biotechnology Inc
Merck & Co Inc
CanSino Biologics Inc
Dynavax Technologies Corp
Celltrion Inc
AstraZeneca Plc
Pfizer Inc
Novavax Inc
Enanta Pharmaceuticals Inc
Arcturus Therapeutics Holdings Inc
Inovio Pharmaceuticals Inc
Valneva SE
Kintor Pharmaceutical Ltd
Bavarian Nordic A/S
Eiger BioPharmaceuticals Inc
Sanofi
Curevac NV
Relief Therapeutics Holding AG
Shionogi & Co Ltd
Shionogi & Co Ltd
Gilead Sciences Inc
Humanigen Inc
Atea Pharmaceuticals Inc
Aridis Pharmaceuticals Inc
Novavax Inc
Eli Lilly and Co
Celularity Inc
Avalo Therapeutics Inc
Ionis Pharmaceuticals Inc
InflaRx NV
Vaxart Inc
Edesa Biotech Inc
Immunome Inc
Shionogi & Co Ltd
Atossa Therapeutics Inc
Brii Biosciences Ltd
Histogen Inc
I-Mab
RedHill Biopharma Ltd
First Wave BioPharma Inc
TFF Pharmaceuticals Inc
Shionogi & Co Ltd
Atossa Therapeutics Inc
Sinovac Biotech Ltd
Table of Contents
Frequently asked questions
-
Which are some of the key COVID-19 prophylactic vaccines?
Some of the key COVID-19 prophylactic vaccines are Comirnaty, Spikevax, Nuvaxovid, Vaxzevria, COVID-19 Vaccine, VidPrevtyn Beta, VLA-2001, Convidecia, Covaxin, and Coronavirus Disease 2019 (COVID-19) vaccine.
-
Which are some of the key COVID-19 therapeutic drugs?
Some of the key COVID-19 therapeutic drugs are Paxlovid, Veklury, Regen-Cov, Lagevrio, Evusheld, Bamlanivimab/Etesevimab, Xevudy, Bebtelovimab, Amubarvimab + Romlusevimab, and Regkirona.
-
Which are the top three marketed COVID-19 drugs?
The top three marketed COVID-19 forecast drugs include two prophylactic vaccines – Comirnaty and Spikevax – and one therapeutic drug – Paxlovid.
-
Which drug is expected to achieve peak forecast sales in 2022?
The forecast sales of Paxlovid are expected to peak in 2022 due to its approval at the end of 2021.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Pharmaceuticals reports







