Coronary Artery Bypass Graft Surgery Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Coronary Artery Bypass Graft Surgery Pipeline Products Market Report Overview
Coronary artery bypass graft surgery devices are used in the surgery to improve blood flow to the obstructed coronary artery. Surgeons use these devices to treat people who have severe coronary heart disease. The Coronary Artery Bypass Graft Surgery pipeline market research report provides comprehensive information about the Coronary Artery Bypass Graft Surgery pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
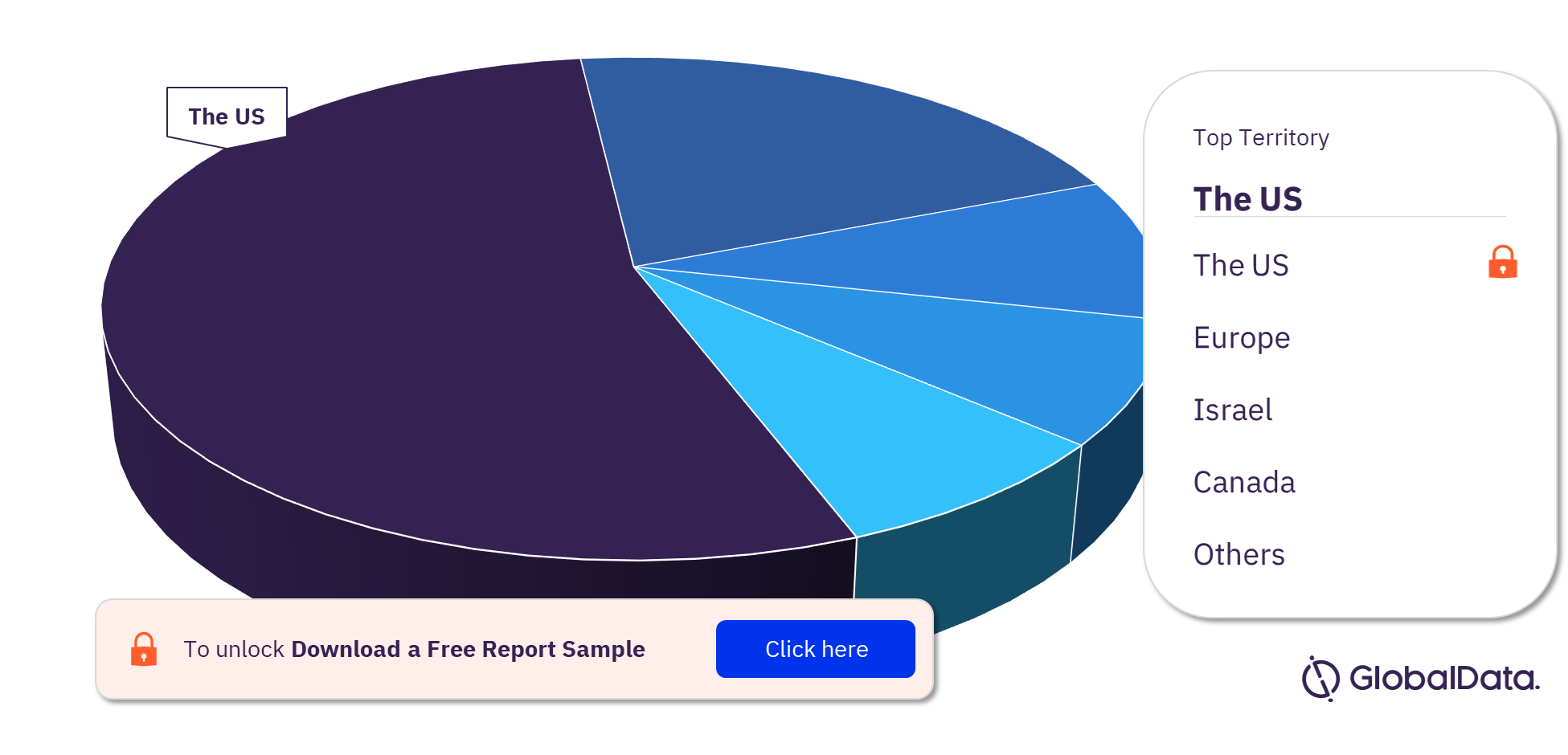
Coronary Artery Bypass Graft Surgery Pipeline Products Market Segmentation by Territories
The key territories with products in the pipeline are the US, Europe, Israel, and Canada. As of February 2023, the US has the highest number of products in the pipeline out of them all.
Coronary Artery Bypass Graft Surgery Pipeline Products Market Analysis, by Territories, 2023 (%)
For more territory insights into the Coronary Artery Bypass Graft Surgery pipeline products market, download a free report sample
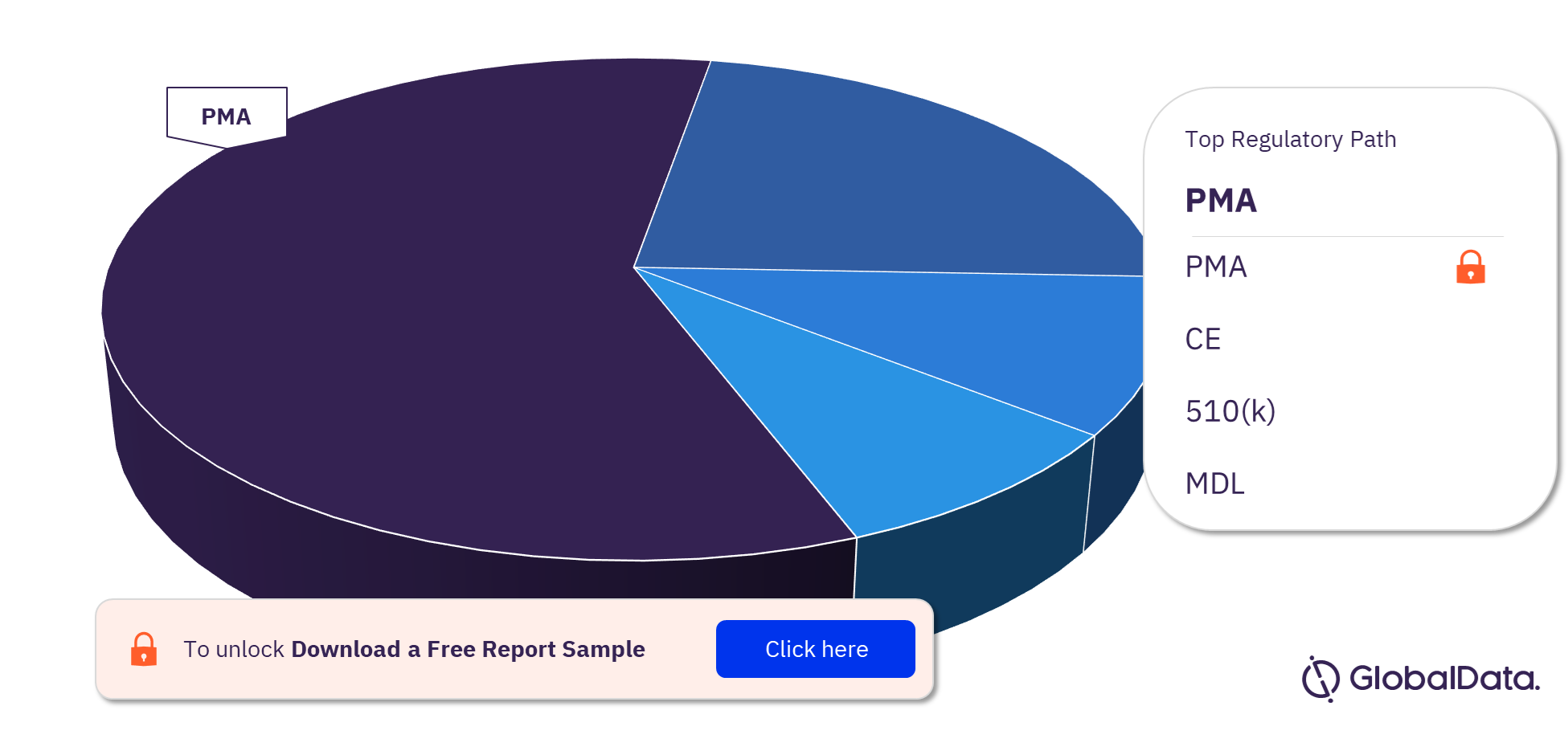
Coronary Artery Bypass Graft Surgery Pipeline Products Market Segmentation by Regulatory Paths
The key regulatory paths followed by the Coronary Artery Bypass Graft Surgery pipeline products market are PMA, CE, 510(k), and MDL. Most of the products follow the PMA pathway to enter the market.
Coronary Artery Bypass Graft Surgery Pipeline Products Market Analysis, by Regulatory Paths, 2023 (%)
For more Coronary Artery Bypass Graft Surgery pipeline products regulatory path insights, download a free report sample
Coronary Artery Bypass Graft Surgery Pipeline Products Market - Competitive Landscape
Some of the leading companies in the Coronary Artery Bypass Graft Surgery pipeline products market are AdvanSource Biomaterials Corporation, ArtiFex Medical GmbH, Axcelon Biopolymers Corp, CardioPolymers Inc, Cardious Inc, Centre Hospitalier Universitaire de Nice, Coromedic Ltd., Cytograft Tissue Engineering Inc (Inactive), Elana bv, and enVVeno Medical Corp.
AdvanSource Biomaterials Corporation: Headquartered in Wilmington, Massachusetts, USA, AdvanSource is a polymer materials company that develops polymer materials used in the design and development of medical devices. AdvanSource’s services include customized materials, product extensions, custom solutions, and project management. The company has a presence in the US, Canada, China, India, and Egypt.
Axcelon Biopolymers Corp: It is an innovative biomaterials company focused on leveraging its unique bacterial nanocellulose platform technology to develop high value products for wound care, medical devices, tissue engineering, and industrial applications. The company has its presence in London, and Canada.
Cardious Inc: Headquartered in Northfield, Minnesota, USA, Cardious is a medical device company that offers valve bypass graft systems. The company offers minimally invasive heart valve therapies. Cardious’ proprietary product aortic valve bypass graft system is a minimally invasive alternative to traditional aortic valve replacement therapy. The company’s valve bypass graft systems are used as beating heart alternative to traditional aortic valve replacement therapy. It caters to cardiac surgeons, hospitals, and medical centers. The company markets its products across the US.
For more company insights into the Coronary Artery Bypass Graft Surgery pipeline products market, download a free report sample
Coronary Artery Bypass Graft Surgery Pipeline Products Market Report Overview
| Key Territories | The US, Europe, Israel, and Canada |
| Key Regulatory Paths | PMA, CE, 510(k), and MDL |
| Leading Companies | AdvanSource Biomaterials Corporation, ArtiFex Medical GmbH, Axcelon Biopolymers Corp, CardioPolymers Inc, Cardious Inc, Centre Hospitalier Universitaire de Nice, Coromedic Ltd., Cytograft Tissue Engineering Inc (Inactive), Elana bv, and enVVeno Medical Corp |
Scope
This report provides an in-depth analysis of:
- Coronary artery bypass graft surgery devices under development
- Details of major pipeline products which includes product description, licensing and collaboration details, and other developmental activities
- Major players involved in the development of coronary artery bypass graft surgery devices and lists all their pipeline projects
- Pipeline products based on various stages of development ranging from early development to approved/issued stage
- Key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with a potentially strong product portfolio and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of Coronary Artery Bypass Graft Surgery under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
ArtiFex Medical GmbH
Axcelon Biopolymers Corp
CardioPolymers Inc
Cardious Inc
Centre Hospitalier Universitaire de Nice
Coromedic Ltd.
Cytograft Tissue Engineering Inc (Inactive)
Elana bv
enVVeno Medical Corp
Graft-Loc Inc.
H. Lee Moffitt Cancer Center & Research Institute Inc
iiTech BV
McGill University
Medical 21 Inc
Myocardial Assist Systems and Technology LLC
Neograft Technologies Inc
PetVivo Holdings Inc
Prekubator TTO
RegenMedTX LLC
SeamVad Ltd.
Surg Solutions SL
Synovis Micro Companies Alliance Inc
Thoratec LLC
Tissue Regeneration Technologies LLC
University College London
University of Arizona
University of California Davis
University of California Los Angeles
Xeltis AG
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key territories in the Coronary Artery Bypass Graft Surgery pipeline products market?
The US, Europe, Israel, and Canada are the key territories with products in the pipeline.
-
What are the key regulatory paths of the Coronary Artery Bypass Graft Surgery pipeline products market?
The key regulatory paths followed by the Coronary Artery Bypass Graft Surgery pipeline products market are PMA, CE, 510(k), and MDL.
-
What are the leading companies in the Coronary Artery Bypass Graft Surgery pipeline products market?
Some of the leading companies in the Coronary Artery Bypass Graft Surgery pipeline products market are AdvanSource Biomaterials Corporation, ArtiFex Medical GmbH, Axcelon Biopolymers Corp, CardioPolymers Inc, Cardious Inc, Centre Hospitalier Universitaire de Nice, Coromedic Ltd., Cytograft Tissue Engineering Inc (Inactive), Elana bv, and enVVeno Medical Corp.
-
What are Coronary Artery Bypass Graft Surgery devices?
Coronary Artery Bypass Graft Surgery devices are used in the surgery to improve blood flow to the obstructed coronary artery.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Coronary Artery Bypass Graft Surgery reports