Cartilage Repair Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Cartilage Repair Pipeline Market Report Overview
Cartilage repair procedures aim to regenerate, restore or replace articular cartilage to reduce joint pain and to prevent the development of arthritis. Cartilage repair interventions can occur early in the spectrum of joint damage and offer an opportunity for patients to delay or altogether avoid needing total joint replacement surgery.
The cartilage repair pipeline market research report provides comprehensive information about the cartilage repair pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · Extracellular Matrix Implants
· Intact Tissue Implants · Osteochondral Allograft |
| Key Territories | · United States
· Europe · China · Australia · Canada · Singapore · South Korea · Taiwan · Others |
| Key Regulatory Paths | · 510(k)
· CE · PMA · NMPA · Ninsho · TGA · UKCA |
| Leading Companies | · 3D Bio Corp
· Acro Biomedical Co Ltd · Aesculap Biologics LLC · Anika Therapeutics Inc · Biomimedica, Inc. · Cartilage Inc · Gunze Ltd · Histogen Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Cartilage Repair Pipeline Market by Segments
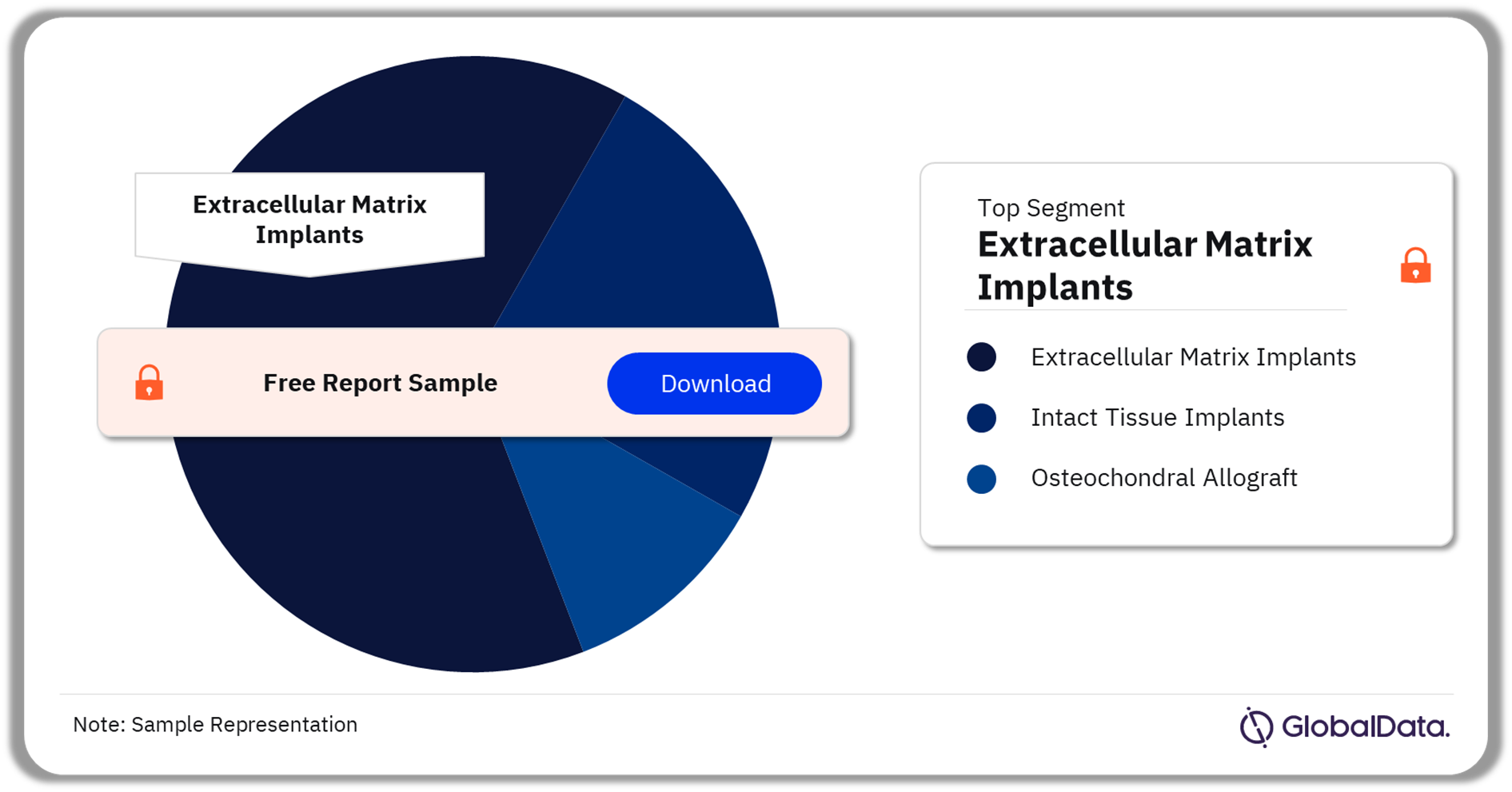
The key segments in the cartilage repair pipeline market are extracellular matrix implants, intact tissue implants, osteochondral allograft. As of August 2023, extracellular matrix implants have the highest number of pipeline products. These implants are decellularized implants that match or mimic the 3D structure of cartilage. They provide endogenous stem cells (chondrocytes) both a scaffold on which to grow, and a full complement of extracellular growth signals. This method of cartilage repair relies upon the stimulation of endogenous stem cells to restore damaged or lost cartilage.
Cartilage Repair Pipeline Market Analysis by Segments, 2023 (%)
Buy full report for insights on cartilage repair pipeline market segments
Cartilage Repair Pipeline Market Segmentation by Territories
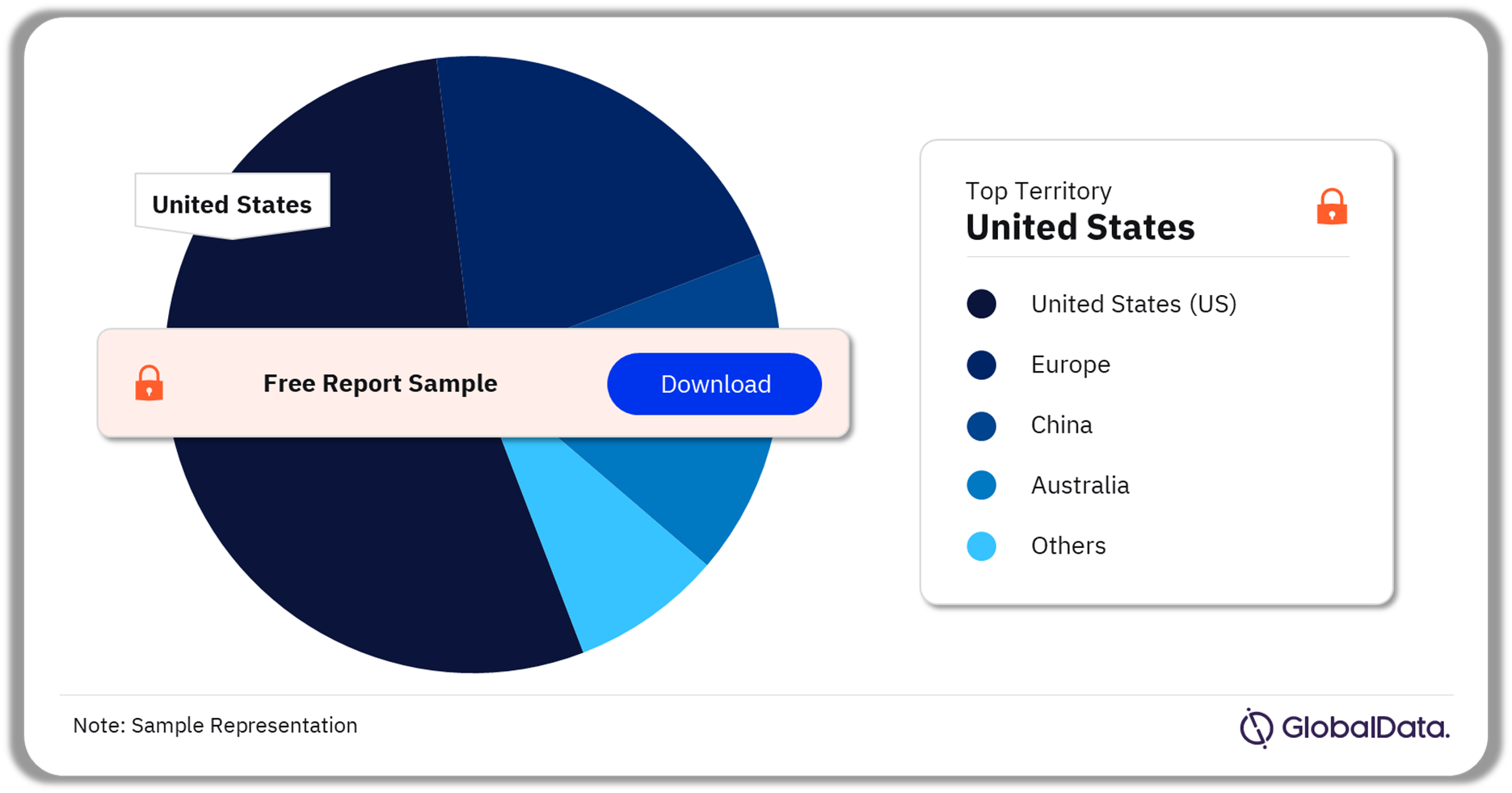
Some of the key territories in the cartilage repair market are the United States (US), Europe, China, Australia, Canada, Singapore, and Taiwan among others. As of August 2023, the US has the highest number of pipeline products.
Cartilage Repair Pipeline Market Analysis by Territories, 2023 (%)
Buy full report with detailed territory insights of the cartilage repair pipeline market
Cartilage Repair Pipeline Market Segmentation by Regulatory Paths
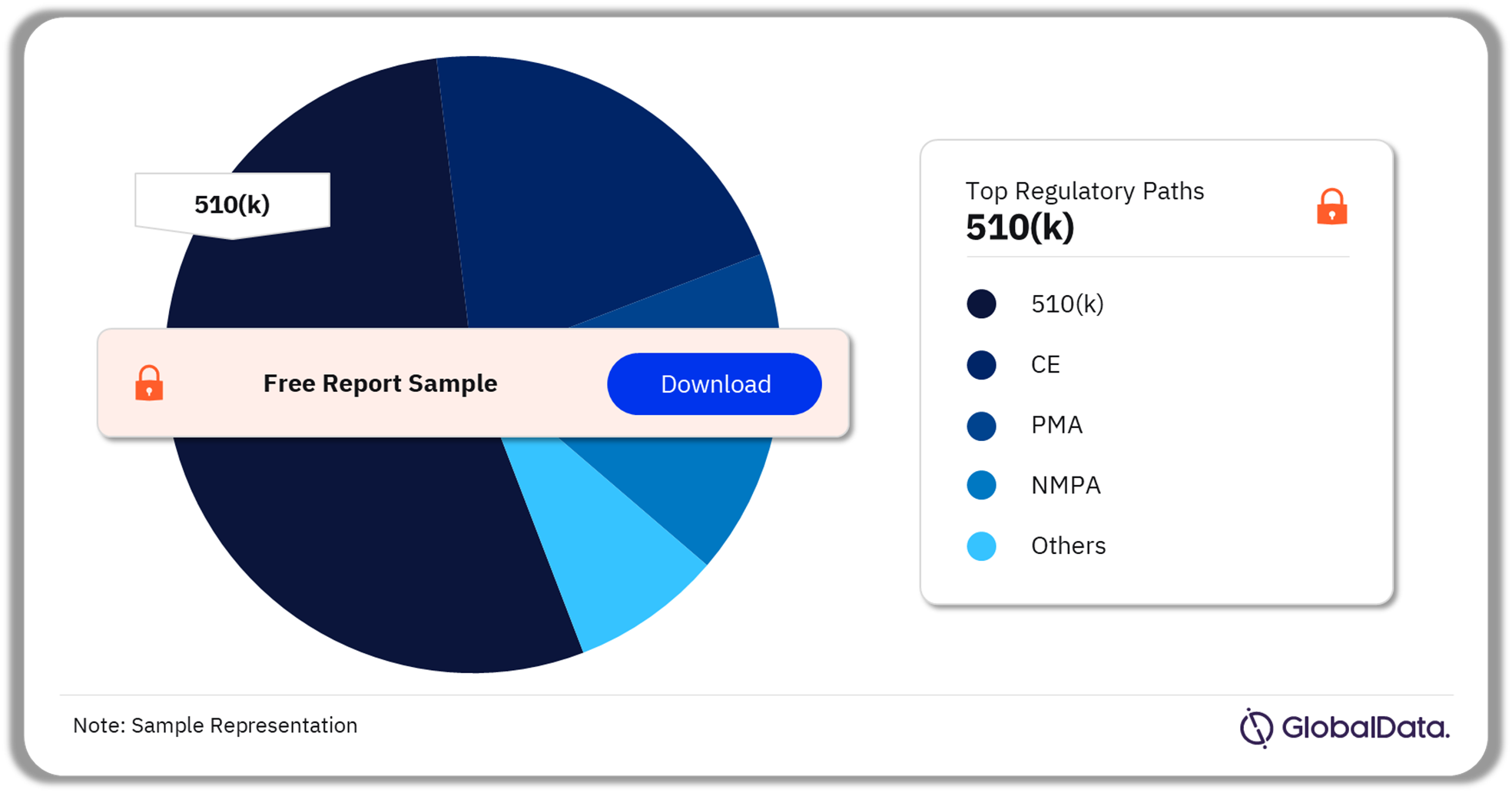
Some of the key regulatory paths in the cartilage repair pipeline market are 510(k), CE, PMA, NMPA, Ninsho, TGA, and UKCA among others. As of August 2023, 510(k) is the most followed pathway for pipeline products in the market.
Cartilage Repair Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy full report for more regulatory path insights into the cartilage repair pipeline market
Cartilage Repair Pipeline Market – Competitive Landscape
Some of the key companies in the cartilage repair pipeline market are 3D Bio Corp, Biomimedica, Inc., Forsagen LLC, Gunze Ltd, Histogen Inc, Lamina Therapeutics, Meidrix GmbH, and Nanochon LLC among others.
3D Bio Corp: The company is a biologics and bioprinting company. The company operates as a biotechnology company that specializes in regenerative medicine and devices. The company’s pipeline products include AuriNovo, AnnuNovo, DiscNovo and NasaNovo. The company provides bio printing platform which focuses on whole tissue living implants for near term indications.
Gunze Ltd: The company manufactures and supplies plastic films, engineering plastics, electronic components, innerwear, leg wear, house casual wear and other products. The company also offers various services such as insurance agency business services, engineering business services, landscaping and greening business services, and sports club operational services.
Cartilage Repair Pipeline Market Analysis by Companies, 2023
Download a free report sample and buy a full report for more company insights into the cartilage repair pipeline market
Segments Covered in the Report
Cartilage Repair Pipeline Market Segments Outlook
- Extracellular Matrix Implants
- Intact Tissue Implants
- Osteochondral Allograft
Cartilage Repair Pipeline Market Territories Outlook
- The US
- Europe
- Australia
- China
- South Africa
- South Korea
Cartilage Repair Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- TGA
- NIFDS
- NMPA
- PMA
Scope
This report provides:
- Extensive coverage of the cartilage repair under development.
- Details of major pipeline products which includes product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of cartilage repair and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of cartilage repair under development
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Acro Biomedical Co Ltd
Aesculap Biologics LLC
Anika Therapeutics Inc
Askel Healthcare Ltd
Biomimedica, Inc.
Cartilage Inc
CoreSpine Technologies LLC
Department of Biomedical Engineering Columbia University
Forsagen LLC
Gunze Ltd
Hainan Susheng Biological Technology Co Ltd
Histogen Inc
Hy2Care BV
Hyalex Orthopaedics Inc
Isto Biologics
Kytogenics Pharmaceuticals Inc
L&C Bio
Lamina Therapeutics
Meidrix GmbH
Nanochon LLC
Novatex Bioengineering SA
Ocugen Inc
Olympus RMS Co Ltd
Regentis Biomaterials Ltd
Rosivo Inc
Royal College of Surgeons Ireland
Ruilin Biomedical Nanjing Co Ltd
Sparta Biomedical Inc
Stemmatters Biotecnologia e Medicina Regenerativa SA
Tetratherix Pty Ltd
University of Alberta
University of Oulu
University of Pennsylvania
Vericel Corp
Zimmer Biomet Holdings Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the cartilage repair pipeline market?
Extracellular matrix implants are the leading segment in the cartilage repair pipeline market in August 2023.
-
Which territory has the highest number of pipeline products in the cartilage repair pipeline market?
As of August 2023, the US has the highest number of products in the cartilage repair pipeline market.
-
Which is the most followed regulatory pathway in the cartilage repair pipeline market?
As of August 2023, 510(k) is the most followed pathway for products in the cartilage repair pipeline market.
-
Who are the major companies operating in the cartilage repair pipeline market?
Some of the key companies in the cartilage repair pipeline market are 3D Bio Corp, Biomimedica, Inc., Forsagen LLC, Gunze Ltd, Histogen Inc, Lamina Therapeutics, Meidrix GmbH, and Nanochon LLC.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Cartilage Repair reports