Cardiovascular Surgery Devices – Pipeline Products by Stage of Development 25
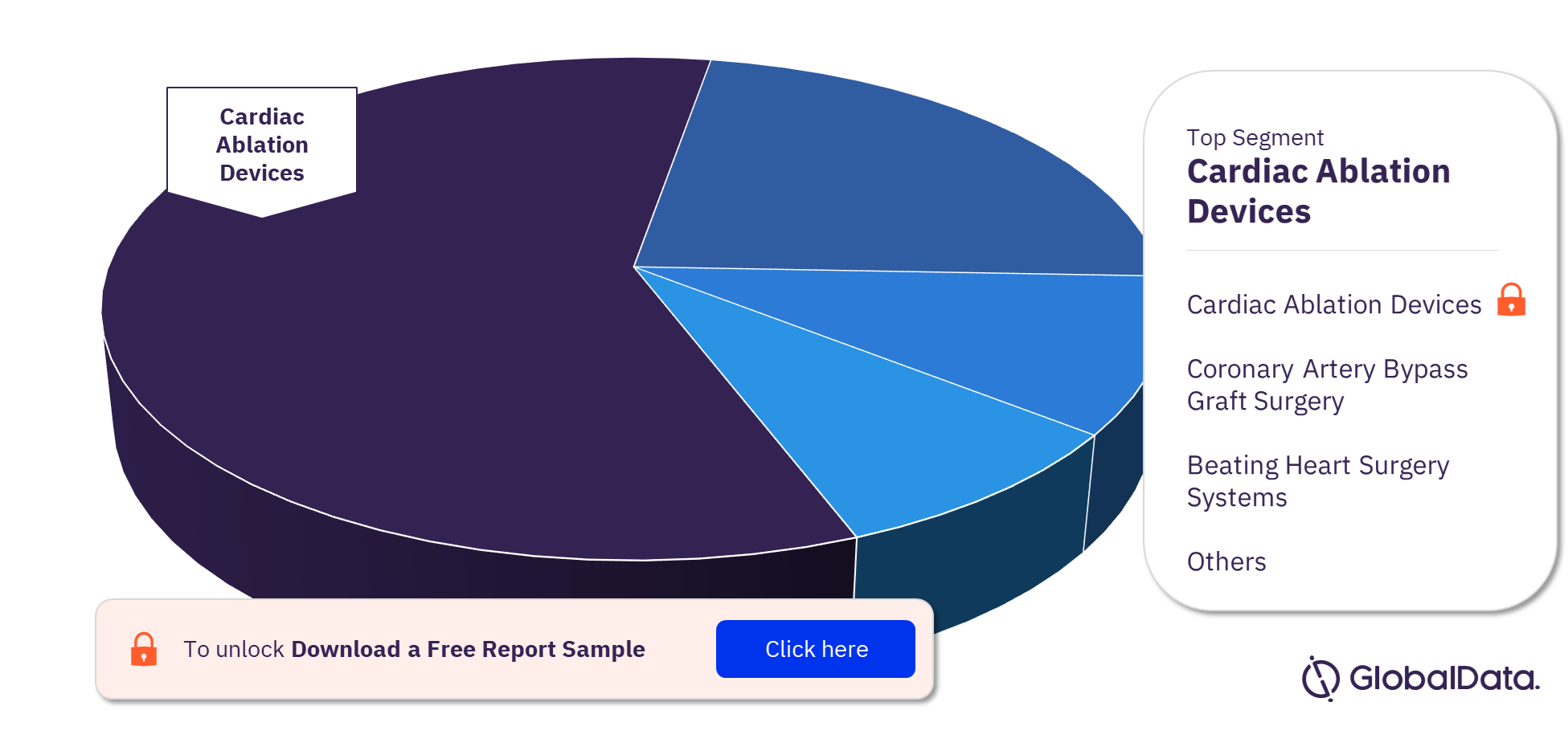
Cardiovascular Surgery Devices – Pipeline Products by Segment 26
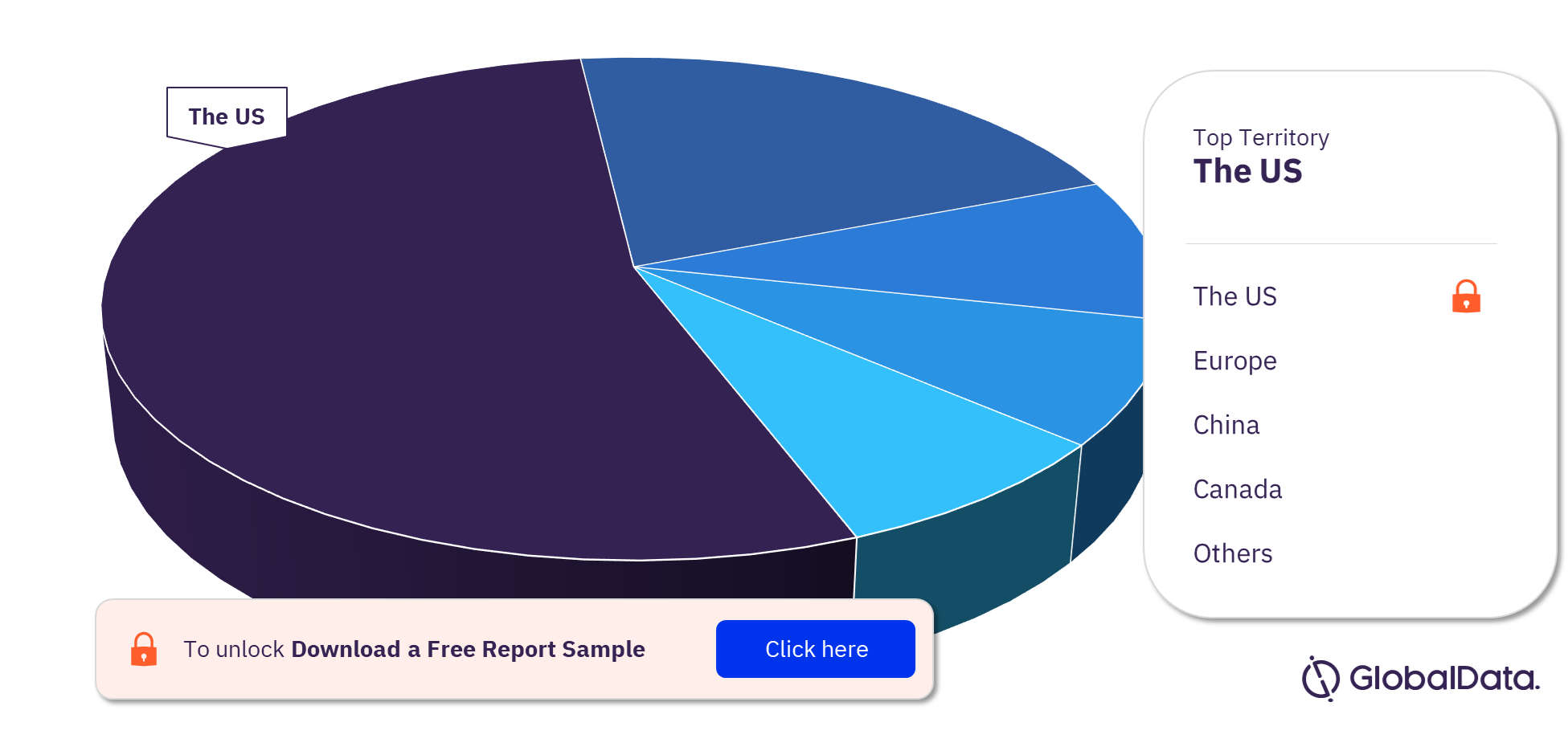
Cardiovascular Surgery Devices – Pipeline Products by Territory 27
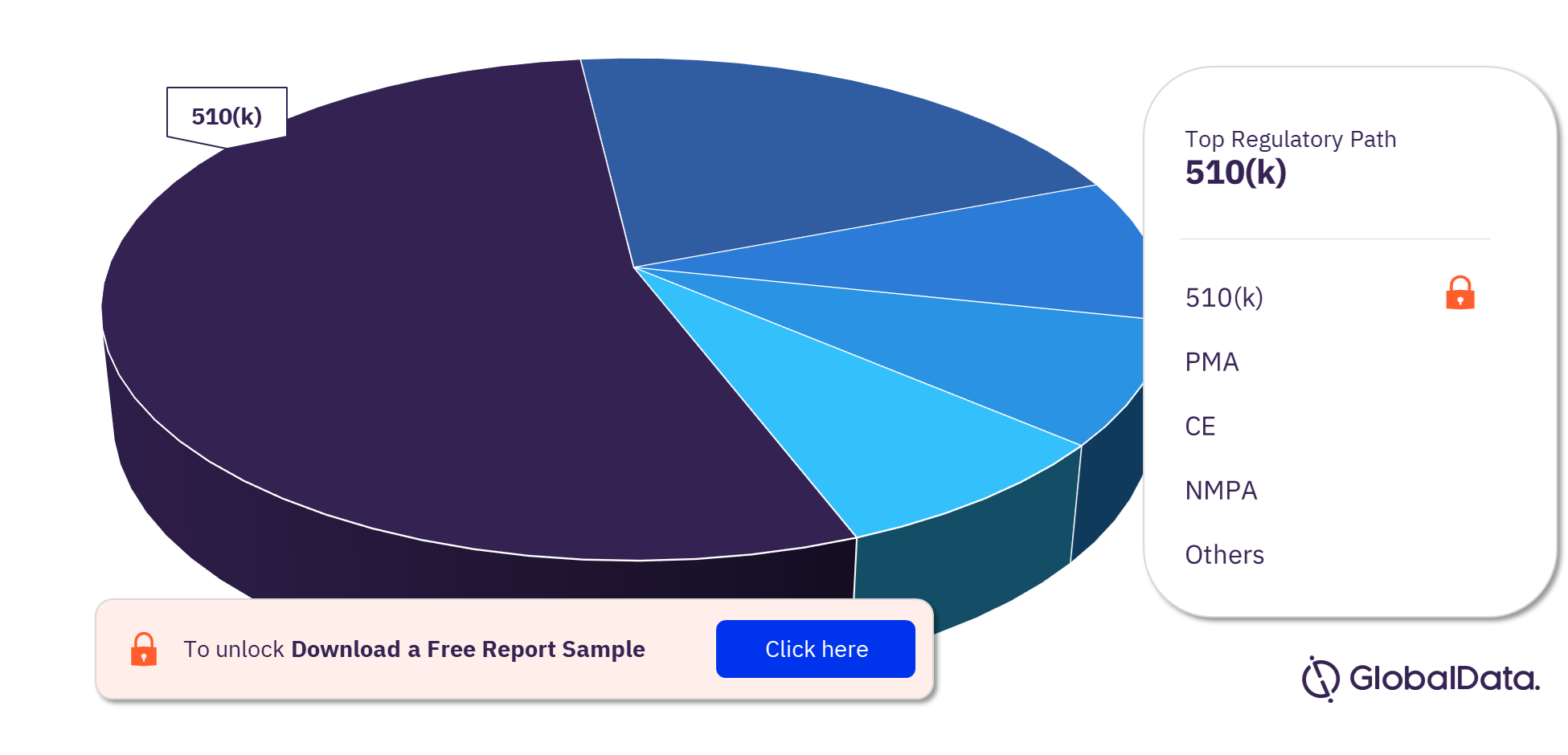
Cardiovascular Surgery Devices – Pipeline Products by Regulatory Path 28
Cardiovascular Surgery Devices – Pipeline Products by Estimated Approval Date 29
Cardiovascular Surgery Devices – Ongoing Clinical Trials 30
Cardiovascular Surgery Devices Companies – Pipeline Products by Stage of Development 31
Cardiovascular Surgery Devices – Pipeline Products by Stage of Development 36
3DT Holdings LLC Pipeline Products & Ongoing Clinical Trials Overview 41
Venous Pressure Preconditioning Device – Product Status 41
Venous Pressure Preconditioning Device – Product Description 41
510 Kardiac Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 42
Lim Transseptal System – Product Status 42
Lim Transseptal System – Product Description 42
Abiomed Inc Pipeline Products & Ongoing Clinical Trials Overview 43
Expandable Sheath – Heart Failure – Product Status 43
Expandable Sheath – Heart Failure – Product Description 43
AblaCor Medical Corporation Pipeline Products & Ongoing Clinical Trials Overview 44
AutoBlator – Product Status 44
AutoBlator – Product Description 44
CircumBlator AF Catheter Ablation System – Product Status 45
CircumBlator AF Catheter Ablation System – Product Description 45
AccuPulse Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 46
PFA Pulsed Electric Field Ablation System – Product Status 46
PFA Pulsed Electric Field Ablation System – Product Description 46
Transseptal Kit – Product Status 47
Transseptal Kit – Product Description 47
Acutus Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 48
AcQBlate Force Sensing Ablation Catheter – Product Status 48
AcQBlate Force Sensing Ablation Catheter – Product Description 48
Acutus Medical Inc – Ongoing Clinical Trials Overview 49
AcQBlate Force Sensing Ablation Catheter – The Pulsed Field Ablation System Study for Atrial Fibrillation (PFA-AF) 50
Adagio Medical Inc. Pipeline Products & Ongoing Clinical Trials Overview 51
iCLAS System – Product Status 51
iCLAS System – Product Description 51
PFCA System – Product Status 52
PFCA System – Product Description 52
Adagio Medical Inc. – Ongoing Clinical Trials Overview 53
iCLAS System – A First-in-human Study of Pulsed Field Cryoablation for the Treatment of Patients with Atrial Fibrillation 54
iCLAS System – Cryoablation for Monomorphic Ventricular Tachycardia Early Feasibility Study (EFS) 54
iCLAS System – iCLAS Cryoablation System Post-Market Clinical Follow-up (PMCF) Study 54
iCLAS System – iCLAS for Persistent Atrial Fibrillation 55
iCLAS System – PARALELL – Pulsed Field Ablation and Pulsed Field CRyoAbLation in PErsistent AtriaL FibrilLation 55
iCLAS System – vCLAS™ Cryoablation for Monomorphic Ventricular Tachycardia 55
PFCA System – PARALELL – Pulsed Field Ablation and Pulsed Field CRyoAbLation in PErsistent AtriaL FibrilLation 56
Advanced Catheter Therapies Inc Pipeline Products & Ongoing Clinical Trials Overview 57
Thrombectomy Device – Product Status 57
Thrombectomy Device – Product Description 57
AdvanSource Biomaterials Corporation Pipeline Products & Ongoing Clinical Trials Overview 58
CardioPass – Product Status 58
CardioPass – Product Description 58
Affera Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Affera Mapping and Ablation System – Product Status 59
Affera Mapping and Ablation System – Product Description 59
HexaGEN Ablation System – Product Status 60
HexaGEN Ablation System – Product Description 60
Sphere9 Catheter – Product Status 60
Sphere9 Catheter – Product Description 61
SpherePVI – Product Status 61
SpherePVI – Product Description 61
Affera Inc – Ongoing Clinical Trials Overview 62
Sphere9 Catheter – Study to Evaluate the Safety and Efficacy of Lattice Sphere Catheter to Reduce Complication Risk During Cardiac Ablation 63
Affera Mapping and Ablation System – First-in-human Study Evaluating the Safety and Effectiveness of the SpherePVI Catheter and Affera System in Patients with Atrial Fibrillation 64
SpherePVI – A Safety and Performance Assessment of the Affera SpherePVI Multi-Ablation System to Treat Paroxysmal Atrial Fibrillation 65
SpherePVI – A Safety and Performance Assessment of the SpherePVI Catheter and the Affera Mapping and Ablation System to Treat Paroxysmal Atrial Fibrillation 65
SpherePVI – First-in-human Study Evaluating the Safety and Effectiveness of the SpherePVI Catheter and Affera System in Patients with Atrial Fibrillation 65
AFreeze GmbH Pipeline Products & Ongoing Clinical Trials Overview 66
Tip-Catheter – Product Status 66
Tip-Catheter – Product Description 66
Amt Medical BV Pipeline Products & Ongoing Clinical Trials Overview 67
ELANA Heart Bypass System – Product Status 67
ELANA Heart Bypass System – Product Description 67
Amt Medical BV – Ongoing Clinical Trials Overview 68
ELANA Heart Bypass System – A First-in-Human (FIH) Study of ELANA Heart Bypass Solution 69
AngioDynamics Inc Pipeline Products & Ongoing Clinical Trials Overview 70
Non-Thermal Vein Ablation Device – Product Status 70
Non-Thermal Vein Ablation Device – Product Description 70
Apaxis Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 71
Anastomotic Suturing Connector – Product Status 71
Anastomotic Suturing Connector – Product Description 71
ApiCor – Product Status 72
ApiCor – Product Description 72
EasyApex CV – Product Status 72
EasyApex CV – Product Description 73
Endo LV – Product Status 73
Endo LV – Product Description 73
Applied Medical Resources Corporation Pipeline Products & Ongoing Clinical Trials Overview 74
Next Generation Cardiac Surgical Device – Product Status 74
Next Generation Cardiac Surgical Device – Product Description 74
APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 75
Pulse Ablation Generator – Product Status 75
Pulse Ablation Generator – Product Description 75
Ring Pulse Ablation Catheter – Product Status 76
Ring Pulse Ablation Catheter – Product Description 76
Arga Medtech SA Pipeline Products & Ongoing Clinical Trials Overview 77
Coherent Sine-Burst Electroporation Ablation System – Product Status 77
Coherent Sine-Burst Electroporation Ablation System – Product Description 77
Non-Thermal Cardiac Ablation System – Product Status 78
Non-Thermal Cardiac Ablation System – Product Description 78
Arga Medtech SA – Ongoing Clinical Trials Overview 79
Coherent Sine-Burst Electroporation Ablation System – Coherent Sine Burst (CSE) Electroporation System Pilot Study in Patients with Atrial Fibrillation 80
ArtiFex Medical GmbH Pipeline Products & Ongoing Clinical Trials Overview 81
ArtiFex Vascular Graft – Product Status 81
ArtiFex Vascular Graft – Product Description 81
Atrian Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 82
mPEF Device – Atrial Fibrillation – Product Status 82
mPEF Device – Atrial Fibrillation – Product Description 82
Atrian Medical Ltd – Ongoing Clinical Trials Overview 83
mPEF Device – Atrial Fibrillation – Atrial Deganglionation as a Therapy for Cardiac Surgery Patients With Atrial Fibrillation 84
mPEF Device – Atrial Fibrillation – Safety and Feasibility of Atrial Deganglionation as Adjunctive Therapy for Atrial Fibrillation 84
AtriCure Inc Pipeline Products & Ongoing Clinical Trials Overview 85
AtriClip System – Stroke – Product Status 85
AtriClip System – Stroke – Product Description 85
AtriCure Bipolar System – Product Status 86
AtriCure Bipolar System – Product Description 86
AtriCure Isolator Synergy Surgical Ablation System – Inappropriate Sinus Tachycardia (IST) – Product Status 86
AtriCure Isolator Synergy Surgical Ablation System – Inappropriate Sinus Tachycardia (IST) – Product Description 87
AtriCure Inc – Ongoing Clinical Trials Overview 88
AtriClip System – Stroke – Stand-alone Thoracoscopic Epicardial Left Atrial Appendage Occlusion with AtriClip Device for Thromboembolism Prevention in Nonvalvular Atrial Fibrillation – the Polish Nationwide Registry 89
AtriCure Bipolar System – Combined Endoscopic Epicardial and Percutaneous Endocardial Ablation Versus Repeated Catheter Ablation in Persistent and Longstanding Persistent Atrial Fibrillation 90
Axcelon Biopolymers Corp Pipeline Products & Ongoing Clinical Trials Overview 91
BNC – PVA Based Vascular Graft – Product Status 91
BNC – PVA Based Vascular Graft – Product Description 91
Axon Therapies Inc Pipeline Products & Ongoing Clinical Trials Overview 92
Satera Ablation System – Product Status 92
Satera Ablation System – Product Description 92
Axon Therapies Inc – Ongoing Clinical Trials Overview 93
Satera Ablation System – Endovascular Ablation of the Right Greater Splanchnic Nerve in Endovascular Ablation of the Right Greater Splanchnic Nerve in Subjects Having HFpEF: Feasibility Study: Randomized Controlled Feasibility Trial – Rebalance-HF Study 94
Baylis Medical Company Inc Pipeline Products & Ongoing Clinical Trials Overview 95
Cardiac Ablation Mitigating Device – Product Status 95
Cardiac Ablation Mitigating Device – Product Description 95
Beth Israel Deaconess Medical Center Pipeline Products & Ongoing Clinical Trials Overview 96
Pericardial Reinforcement Device – Product Status 96
Pericardial Reinforcement Device – Product Description 96
Tissue Transplant And Regeneration Device – Product Status 97
Tissue Transplant And Regeneration Device – Product Description 97
Biosense Webster Inc Pipeline Products & Ongoing Clinical Trials Overview 98
VARIPULSE Catheter – Product Status 98
VARIPULSE Catheter – Product Description 98
Biosense Webster Inc – Ongoing Clinical Trials Overview 99
VARIPULSE Catheter – Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation With the BWI IRE Ablation System (AdmIRE) 100
VARIPULSE Catheter – Pulsed Field Ablation (PFA) System for the Treatment of Paroxysmal Atrial Fibrillation (PAF) by Irreversible Electroporation (IRE) 100
Boston Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 101
Heart Patch Deployment System – Product Status 101
Heart Patch Deployment System – Product Description 101
Millimeter-Scale Device – Product Status 102
Millimeter-Scale Device – Product Description 102
Mitral Valve Repair Clip Delivery Device – Product Status 102
Mitral Valve Repair Clip Delivery Device – Product Description 103
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 104
WM NG Sheath – Product Status 104
WM NG Sheath – Product Description 104
CardioPolymers Inc Pipeline Products & Ongoing Clinical Trials Overview 105
Plexisyl-AF – Product Status 105
Plexisyl-AF – Product Description 105
CardioPrecision Ltd Pipeline Products & Ongoing Clinical Trials Overview 106
CardioPrecision Device – Product Status 106
CardioPrecision Device – Product Description 106
CardioScout, LLC Pipeline Products & Ongoing Clinical Trials Overview 107
Minimally Invasive Surgical Device – Product Status 107
Minimally Invasive Surgical Device – Product Description 107
Cardious Inc Pipeline Products & Ongoing Clinical Trials Overview 108
AvA Aortic Valve Bypass Graft System – Product Status 108
AvA Aortic Valve Bypass Graft System – Product Description 108
Cardiovascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 109
IVL System – Product Status 109
IVL System – Product Description 109
catheter precision Inc Pipeline Products & Ongoing Clinical Trials Overview 110
Vessel Closure Device – Product Status 110
Vessel Closure Device – Product Description 110
Centre Hospitalier Universitaire de Nice Pipeline Products & Ongoing Clinical Trials Overview 111
Shark Anastomosis Connector – Product Status 111
Shark Anastomosis Connector – Product Description 111
Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 112
Right Angle Cannula – Product Status 112
Right Angle Cannula – Product Description 112
CircumFix Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 113
Sternal Closure Device – Product Status 113
Sternal Closure Device – Product Description 113
Corazon Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 114
Beating Heart Aortic Valve Repair Demineralization System – Product Status 114
Beating Heart Aortic Valve Repair Demineralization System – Product Description 114
Corfigo Inc Pipeline Products & Ongoing Clinical Trials Overview 115
HeArTPAD – Product Status 115
HeArTPAD – Product Description 115
Coridea, LLC Pipeline Products & Ongoing Clinical Trials Overview 116
Cardiac Sympathetic Denervation Device – Product Status 116
Cardiac Sympathetic Denervation Device – Product Description 116
Coromedic Ltd. Pipeline Products & Ongoing Clinical Trials Overview 117
Distal Anastomosis Device – Product Status 117
Distal Anastomosis Device – Product Description 117
Proximal Anastomosis Device – Product Status 118
Proximal Anastomosis Device – Product Description 118
CPSI Biotech Pipeline Products & Ongoing Clinical Trials Overview 119
ICEolate Catheter – Product Status 119
ICEolate Catheter – Product Description 120
ICEolate Catheter With Spot Ablation Tip – Product Status 120
ICEolate Catheter With Spot Ablation Tip – Product Description 120
ICEolate Surgical Probe With Control Handle – Product Status 121
ICEolate Surgical Probe With Control Handle – Product Description 121
ICEolate Surgical Probe With Flexible Ablation Tip – Product Status 121
ICEolate Surgical Probe With Flexible Ablation Tip – Product Description 122
Cryofocus Medtech Shanghai Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 123
AF Cryoablation System – Product Status 123
AF Cryoablation System – Product Description 123
Cryo-RDN System – Product Status 124
Cryo-RDN System – Product Description 124
Pulmonary Hypertension Cryoablation System – Product Status 124
Pulmonary Hypertension Cryoablation System – Product Description 125
Cytograft Tissue Engineering Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 126
Cytograft Device – Percutaneous Bypass – Product Status 126
Cytograft Device – Percutaneous Bypass – Product Description 126
LifeLine TEBV – CABG – Product Status 127
LifeLine TEBV – CABG – Product Description 127
Daidalos Solutions BV Pipeline Products & Ongoing Clinical Trials Overview 128
Repair Rings Applicator – Product Status 128
Repair Rings Applicator – Product Description 128
Sutureless Cannula – Product Status 129
Sutureless Cannula – Product Description 129
Sutureless Natural Vascular Connector – Product Status 129
Sutureless Natural Vascular Connector – Product Description 130
Sutureless Vascular Synthetic Graft Connector – Product Status 130
Sutureless Vascular Synthetic Graft Connector – Product Description 130
Edwards Lifesciences Corp Pipeline Products & Ongoing Clinical Trials Overview 131
APTURE – Product Status 131
APTURE – Product Description 131
ESSANA – Product Status 132
ESSANA – Product Description 132
FORETELL – Product Status 132
FORETELL – Product Description 132
Next-Gen EndoVent With Pacing – Product Status 133
Next-Gen EndoVent With Pacing – Product Description 133
Next-Gen QuickDraw Cannula – Product Status 133
Next-Gen QuickDraw Cannula – Product Description 133
Sutrafix – Product Status 134
Sutrafix – Product Description 134
Elana bv Pipeline Products & Ongoing Clinical Trials Overview 135
ELANA CLIP 3.0 – Product Status 135
ELANA CLIP 3.0 – Product Description 135
Emory University Pipeline Products & Ongoing Clinical Trials Overview 136
CorAccess – Product Status 136
CorAccess – Product Description 136
Endoron Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 137
Zeroneck – Abdominal Aortic Aneurysm – Product Status 137
Zeroneck – Abdominal Aortic Aneurysm – Product Description 137
enVVeno Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 138
CoreoGraft – Product Status 138
CoreoGraft – Product Description 138
enVVeno Medical Corp – Ongoing Clinical Trials Overview 139
CoreoGraft – First-in-human Trial Evaluating the Efficacy of CoreoGraft in the Coronary Artery Bypass Graft (CABG) Surgery 140
Epiendoaf Inc Pipeline Products & Ongoing Clinical Trials Overview 141
EpiEndoAF Device – Product Status 141
EpiEndoAF Device – Product Description 141
EpiEP, Inc. Pipeline Products & Ongoing Clinical Trials Overview 142
Epicardial Access Trocar – Product Status 142
Epicardial Access Trocar – Product Description 142
Excision Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 143
Leaflet Modification System – Product Status 143
Leaflet Modification System – Product Description 143
Farapulse Inc Pipeline Products & Ongoing Clinical Trials Overview 144
Endo Faraflex – Product Status 144
Endo Faraflex – Product Description 144
Endo Farapoint – Product Status 145
Endo Farapoint – Product Description 145
Epi Faraone – Product Status 145
Epi Faraone – Product Description 146
FARAPULSE PFA System – Product Status 146
FARAPULSE PFA System – Product Description 146
Next Gen FARAWAVE Catheter – Product Status 147
Next Gen FARAWAVE Catheter – Product Description 147
Farapulse Inc – Ongoing Clinical Trials Overview 148
FARAPULSE PFA System – A Prospective Randomized Pivotal Trial of the FARAPULSE Pulsed Field Ablation System Compared with Standard of Care Ablation in Patients with Paroxysmal Atrial Fibrillation 149
FARAPULSE PFA System – A Prospective Single Arm Open Label Study of the FARAPULSE Pulsed Field Ablation System in Subjects with Persistent Atrial Fibrillation 149
FARAPULSE PFA System – A Real-world Study of the FARAPULSE Pulsed Field Ablation System in A Chinese Population With Paroxysmal Atrial Fibrillation 149
FARAPULSE PFA System – PersAFOne II(Pronounced Per-‘Se-fa-ne 2); Feasibility Study of the FARAPULSE Cardiac Ablation System Plus in the Treatment of Persistent Atrial Fibrillation 150
FARAPULSE PFA System – PersAFOne III: Feasibility Study of the FARAPULSE Pulsed Field Ablation System Plus – PersAF in the Treatment of Persistent Atrial Fibrillation 150
FARAPULSE PFA System – Real World Data Collection in Subjects Treated With the FARAPULSE Pulsed Field Ablation System 150
FARAPULSE PFA System – Single Shot Pulmonary Vein Isolation: Comparison of Cryoballoon vs. Pulsed Field Ablation in Patients With Symptomatic Paroxysmal Atrial Fibrillation – A Multi-Center Non-Inferiority Design Clinical Trial (The SINGLE SHOT CHAMPION Trial) 151
FARAPULSE PFA System – The FARAPULSE FARA-Freedom Trial A Prospective Open Label Single Arm Post Market Clinical Follow-Up Trial of the FARAPULSE Pulsed Field Ablation System in Patients with Paroxysmal Atrial Fibrillation 151
Next Gen FARAWAVE Catheter – The Effect of Pulsed-field and Radiofrequency Ablation for Atrial Fibrillation on Platelet Activity, Coagulation and Inflammation 152
Galaxy Medical Products Inc Pipeline Products & Ongoing Clinical Trials Overview 153
Centauri System – Product Status 153
Centauri System – Product Description 153
Galaxy Medical Products Inc – Ongoing Clinical Trials Overview 154
Centauri System – Study Evaluating the Safety and Efficacy of the Centauri system (ECLIPSE-AF) 155
Centauri System – Study Evaluating the Safety and Efficacy of the Centauri System in Patients with Persistent Atrial Fibrillation: SPACE-AF 155
Graft-Loc Inc. Pipeline Products & Ongoing Clinical Trials Overview 156
Graft-Loc Device – Product Status 156
Graft-Loc Device – Product Description 156
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 157
Vascular Stent – Product Status 157
Vascular Stent – Product Description 157
Hadassah Medical Center Pipeline Products & Ongoing Clinical Trials Overview 158
Cardio Safex – Product Status 158
Cardio Safex – Product Description 158
Qualiblate – Product Status 159
Qualiblate – Product Description 159
Hangzhou Nuocheng Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 160
Liwen RF Ablation System – Product Status 160
Liwen RF Ablation System – Product Description 160
Hangzhou Nuomao Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 161
CardioPulse Field Ablation System – Product Status 161
CardioPulse Field Ablation System – Product Description 161
Hangzhou Nuomao Medical Technology Co Ltd – Ongoing Clinical Trials Overview 162
CardioPulse Field Ablation System – A Prospective, Multicenter, Single-arm Objective Performance Criteria Evaluation of the Safety and Efficacy of a Pulsed Field Ablation System in Patients with Paroxysmal Atrial Fibrillation 163
HeartLander Surgical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 164
HeartLander – Product Status 164
HeartLander – Product Description 164
HeartStitch Inc. Pipeline Products & Ongoing Clinical Trials Overview 165
HeartStitch – Remo Pro – Product Status 165
HeartStitch – Remo Pro – Product Description 165
Helical Solutions, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 166
Catheter-Based Ablation Device – Product Status 166
Catheter-Based Ablation Device – Product Description 166
Huifeng Biotechnology (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 167
Atrial Fibrillation Pulsed Field Ablation System – Product Status 167
Atrial Fibrillation Pulsed Field Ablation System – Product Description 167
iiTech BV Pipeline Products & Ongoing Clinical Trials Overview 168
S² Distal Anastomotic System – Product Status 168
S² Distal Anastomotic System – Product Description 168
S2 Proximal Anastomotic System – Product Status 169
S2 Proximal Anastomotic System – Product Description 169
Insight Lifetech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Pulsed Field Ablation Catheter – Product Status 170
Pulsed Field Ablation Catheter – Product Description 170
Pulsed Field Ablation System – Product Status 171
Pulsed Field Ablation System – Product Description 171
Japan Lifeline Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 172
ALPHA1 Ablation Catheter – Product Status 172
ALPHA1 Ablation Catheter – Product Description 172
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 173
Catheter Guiding Computational Modeling Tool – Product Status 173
Catheter Guiding Computational Modeling Tool – Product Description 173
Kardium Inc Pipeline Products & Ongoing Clinical Trials Overview 174
CHS – Coated Globe Mapping And Ablation System – Product Status 174
CHS – Coated Globe Mapping And Ablation System – Product Description 174
Globe Mapping And Ablation System – Product Status 175
Globe Mapping And Ablation System – Product Description 175
Globe PF System – Product Status 176
Globe PF System – Product Description 176
Kardium Inc – Ongoing Clinical Trials Overview 177
Globe Mapping And Ablation System – A Prospective, Non-randomized Clinical Study to Assess the Performance of the Globe Positioning System for Left Atrial Mapping During Cardiac Ablation for the Treatment of Atrial Fibrillation 178
Globe Mapping And Ablation System – An Investigational Clinical Study of Globe Mapping and Ablation System in Treatment of Patients with Atrial Fibrillation 178
Globe Mapping And Ablation System – Clinical Evaluation of Arrhythmia Mapping With a Globe-Shaped, High-Density, Multi-Electrode Mapping Catheter 178
Globe Mapping And Ablation System – Prospective, Multi-centre, Observational Registry to Evaluate the Clinical Performance of the Globe Mapping and Ablation System for the Treatment of Atrial Fibrillation 179
Globe Mapping And Ablation System – PULSE-EU – A Prospective, Non-randomized Clinical Pilot Study to Assess Safety and Performance of a Pulsed Field Device for Global Mapping and Ablation of the Left Atrium for the Treatment of Atrial Fibrillation 179
Globe PF System – A Prospective, Multicenter Clinical Study to Evaluate the Safety and Effectiveness of the Globe Pulsed Field System for Treating Patients with Symptomatic Paroxysmal or Persistent Atrial Fibrillation 180
LaunchPoint Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 181
Quintessential Ventricular Cannula – Product Status 181
Quintessential Ventricular Cannula – Product Description 181
LeMaitre Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 182
XenoSure Biologic Vascular Patch – Product Status 182
XenoSure Biologic Vascular Patch – Product Description 182
LeMaitre Vascular Inc – Ongoing Clinical Trials Overview 183
XenoSure Biologic Vascular Patch – Random, Controlled, Single-blinded, Multi-center and Non-inferiority Clinical Study to Evaluate Safety and Effectiveness of XenoSure Biological Patch in the Application of Cardiac Repair 184
XenoSure Biologic Vascular Patch – Random, Controlled, Single-blinded, Multi-center and Non-inferiority Clinical Study to Evaluate Safety and Effectiveness of XenoSure Biological Patch in the Application of Peripheral Vascular Repair 184
Lepu Medical Technology (Beijing) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 185
Disposable Delivery Sheath – Product Status 185
Disposable Delivery Sheath – Product Description 185
Disposable Introducing Sheath – Product Status 186
Disposable Introducing Sheath – Product Description 186
Thrombus Protection Device – Product Status 186
Thrombus Protection Device – Product Description 187
LuxCath, LLC Pipeline Products & Ongoing Clinical Trials Overview 188
OmniView Light-Guided Ablation Catheter – Product Status 188
OmniView Light-Guided Ablation Catheter – Product Description 188
McGill University Pipeline Products & Ongoing Clinical Trials Overview 189
Transcatheter Aorta Pulmonary Shunt – Product Status 189
Transcatheter Aorta Pulmonary Shunt – Product Description 189
Medical 21 Inc Pipeline Products & Ongoing Clinical Trials Overview 190
Maverics Graft – Product Status 190
Maverics Graft – Product Description 190
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 191
Cardioblate Irrigated RF (IRF) System – Non-Paroxysmal Atrial Fibrillation – Product Status 191
Cardioblate Irrigated RF (IRF) System – Non-Paroxysmal Atrial Fibrillation – Product Description 192
CryoFlex Surgical Ablation System – Non-Paroxysmal Atrial Fibrillation – Product Status 192
CryoFlex Surgical Ablation System – Non-Paroxysmal Atrial Fibrillation – Product Description 192
PulseSelect PFA System – Product Status 193
PulseSelect PFA System – Product Description 193
Medtronic Plc – Ongoing Clinical Trials Overview 194
Cardioblate Irrigated RF (IRF) System – Non-Paroxysmal Atrial Fibrillation – Irrigated Radio Frequency Ablation to Terminate Non-paroxysmal Atrial Fibrillation: Terminate AF Study 195
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 196
Expandable Sheath – Product Status 196
Expandable Sheath – Product Description 196
Femoral Arterial Cannula – Product Status 197
Femoral Arterial Cannula – Product Description 197
Femoral Venous Cannula – Product Status 197
Femoral Venous Cannula – Product Description 198
Microsurgical Innovations Inc Pipeline Products & Ongoing Clinical Trials Overview 199
Vascular Coupling Device – Product Status 199
Vascular Coupling Device – Product Description 199
Most Cardio LLC Pipeline Products & Ongoing Clinical Trials Overview 200
MIS Port – Cardiovascular Diseases – Product Status 200
MIS Port – Cardiovascular Diseases – Product Description 200
Myocardial Assist Systems and Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 201
Sutureless Anastomotic Graft Connection Device – Product Status 201
Sutureless Anastomotic Graft Connection Device – Product Description 201
National Science and Technology Development Agency Pipeline Products & Ongoing Clinical Trials Overview 202
Cardiac Ablation Device – Product Status 202
Cardiac Ablation Device – Product Description 202
Neograft Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 203
Neovaso – Product Status 203
Neovaso – Product Description 203
Nido Surgical LLC Pipeline Products & Ongoing Clinical Trials Overview 204
Cardioport – Product Status 204
Cardioport – Product Description 204
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 205
Cardiac Tissue Ablation Device – Product Status 205
Cardiac Tissue Ablation Device – Product Description 205
Occlutech International AB Pipeline Products & Ongoing Clinical Trials Overview 206
Occlutech Atrial Flow Regulator (AFR) Device – Product Status 206
Occlutech Atrial Flow Regulator (AFR) Device – Product Description 206
Occlutech International AB – Ongoing Clinical Trials Overview 207
Occlutech Atrial Flow Regulator (AFR) Device – A Multicenter, International, Follow-up Study to Monitor the Efficacy and Safety of the Occlutech Atrial Flow Regulator in Heart Failure Patients 208
Occlutech Atrial Flow Regulator (AFR) Device – Flow Regulation by Opening the SepTum in Patients with Heart Failure; a Prospective, Randomized, Sham-controlled, Double-blind, Global Multicenter Study 208
Occlutech Atrial Flow Regulator (AFR) Device – Pomeranian Atrial Flow Regulator in Congestive Heart Failure (PROLONGER) Trial 208
Occlutech Atrial Flow Regulator (AFR) Device – Prospective, Non-randomized, Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Patients with Pulmonary Hypertension 209
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 210
Expandable Sheath – Product Status 210
Expandable Sheath – Product Description 210
Pavmed Inc Pipeline Products & Ongoing Clinical Trials Overview 211
Caldus – Renal Denervation – Product Status 211
Caldus – Renal Denervation – Product Description 211
Caldus – Traditional Tissue Ablation – Product Status 212
Caldus – Traditional Tissue Ablation – Product Description 212
Peca Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 213
RV-PA Shunt – Product Status 213
RV-PA Shunt – Product Description 213
PeriCor LLC Pipeline Products & Ongoing Clinical Trials Overview 214
PeriPath – Product Status 214
PeriPath – Product Description 214
PetVivo Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 215
Coronary Artery Bypass Graft – Product Status 215
Coronary Artery Bypass Graft – Product Description 215
Prekubator TTO Pipeline Products & Ongoing Clinical Trials Overview 216
Snapcon – Product Status 216
Snapcon – Product Description 216
Protaryx Medical Pipeline Products & Ongoing Clinical Trials Overview 217
Protaryx Device – Product Status 217
Protaryx Device – Product Description 217
Rafael Medical Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 218
Space Station – Product Status 218
Space Station – Product Description 218
RegenMedTX LLC Pipeline Products & Ongoing Clinical Trials Overview 219
Neo-Vessel Replacement – Coronary Artery Bypass – Product Status 219
Neo-Vessel Replacement – Coronary Artery Bypass – Product Description 219
Replication Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 220
Hydrogel Implant – Vascular Surgery – Product Status 220
Hydrogel Implant – Vascular Surgery – Product Description 220
RWTH Aachen University Pipeline Products & Ongoing Clinical Trials Overview 221
StentEx – Product Status 221
StentEx – Product Description 221
SeamVad Ltd. Pipeline Products & Ongoing Clinical Trials Overview 222
MySide – Product Status 222
MySide – Product Description 222
Shanghai Antec Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 223
Cryoballoon Ablation System – Product Status 223
Cryoballoon Ablation System – Product Description 223
Pulsed Electric Field Ablation System – Product Status 224
Pulsed Electric Field Ablation System – Product Description 224
Shanghai HeartCare Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 225
Cryoablation Device – Product Status 225
Cryoablation Device – Product Description 225
Shanghai Keku Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 226
Cardiac Ablation Device – Atrial Fibrillation – Product Status 226
Cardiac Ablation Device – Atrial Fibrillation – Product Description 226
Cardiac Implant – Atrial Fibrillation – Product Status 227
Cardiac Implant – Atrial Fibrillation – Product Description 227
Shanghai Weiqi Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 228
Cardiac Cryoablation System – Product Status 228
Cardiac Cryoablation System – Product Description 228
Shanghai Weiqi Medical Equipment Co Ltd – Ongoing Clinical Trials Overview 229
Cardiac Cryoablation System – A Prospective, Multicenter, Single-arm Clinical Trial Evaluating the Safety and Efficacy of a Cardiac Cryoablation System for the Treatment of Paroxysmal Atrial Fibrillat
![]()