Blood Pressure Monitors Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Blood Pressure Monitors Pipeline Market Overview
Blood pressure monitors include blood pressure transducers. It converts blood pressure into electrical signals and sphygmomanometers, a device consisting of a pressure gauge and a rubber cuff that wraps around the upper arm and wrist which inflates to constrict the arteries and measure the blood pressure.
The Blood Pressure Monitors pipeline market research report provides comprehensive information about the pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · Ambulatory Blood Pressure Monitors
· Wrist Blood Pressure Monitors · Blood Pressure Transducers · Upper Arm Blood Pressure Monitors |
| Key Territories | · The US
· China · Global · Europe · Singapore · Mexico · Others |
| Key Regulatory Paths | · NMPA
· 510(k) · CE · HSA · De novo · MDITAC · TGA |
| Leading Companies | · Aktiia SA
· AtCor Medical Inc · Barron Associates, Inc. · Blumio Inc · Bold Diagnostics LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Blood Pressure Monitors Pipeline Market by Segments
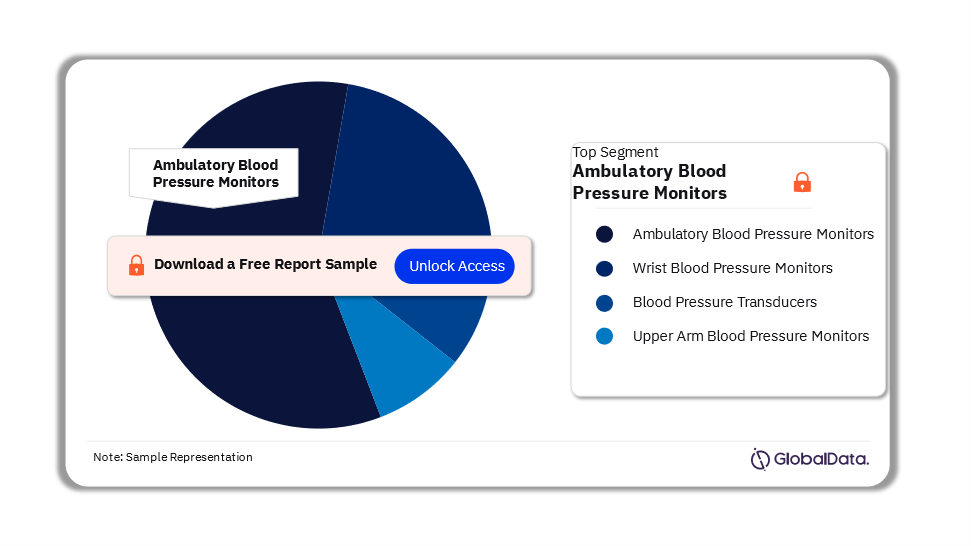
The key segments in the blood pressure monitors market are ambulatory blood pressure monitors, wrist blood pressure monitors, blood pressure transducers, and upper arm blood pressure monitors. As of August 2023, the ambulatory blood pressure monitors segment accounts for the highest number of pipeline products.
Blood Pressure Monitors Pipeline Market Analysis by Segments, 2023 (%)
For more segment insights into the Blood Pressure Monitors pipeline market, download a free report sample
Blood Pressure Monitors Pipeline Market Segmentation by Territories
Some of the key territories in the Blood Pressure Monitors market are the US, China, Europe, Singapore, Mexico, and South Korea among others. As of August 2023, the US accounts for the highest number of Blood Pressure Monitor pipeline products.
Blood Pressure Monitors Pipeline Market Analysis by Territories, 2023 (%)
For more territory insights into the Blood Pressure Monitors pipeline market, download a free report sample
Blood Pressure Monitors Pipeline Market Segmentation by Regulatory Paths
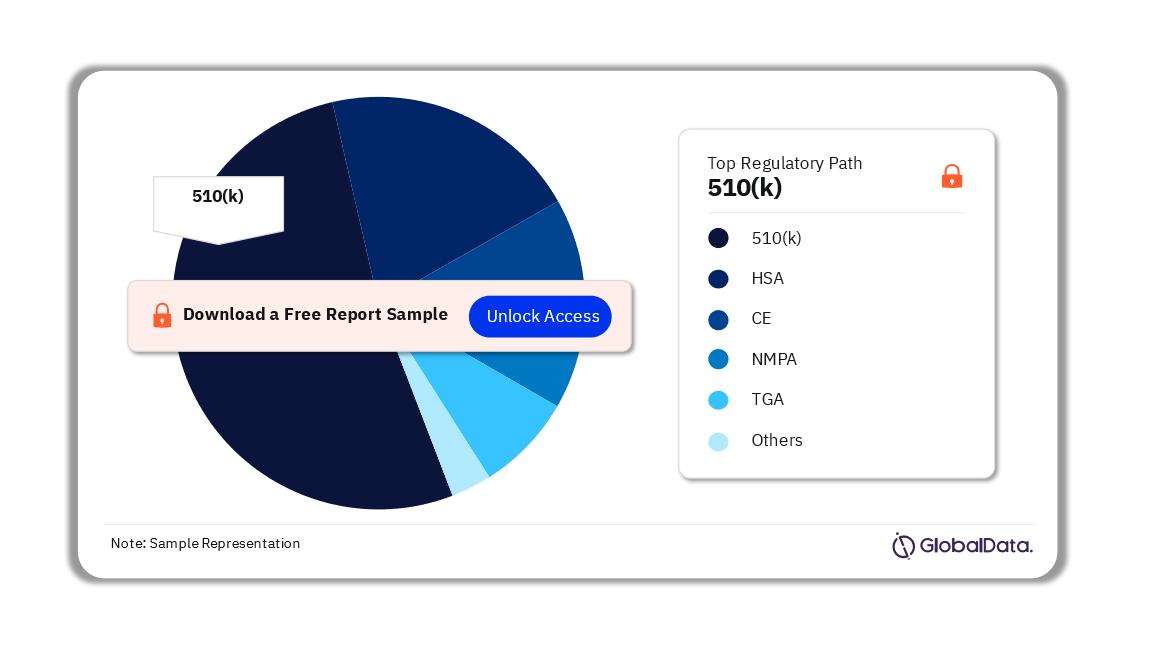
Some of the key regulatory paths in the Blood Pressure Monitors pipeline market are 510(k), NMPA, CE, HSA, De novo, MDITAC, and TGA among others. As of August 2023, 510(k) is the most followed pathway for pipeline products.
Blood Pressure Monitors Pipeline Market Analysis by Regulatory Paths, 2023 (%)
For more regulatory path insights into the Blood Pressure Monitors pipeline market, download a free report sample
Blood Pressure Monitors Pipeline Market – Competitive Landscape
Some of the key companies in the Blood Pressure Monitors pipeline market are Aktiia SA, AtCor Medical Inc, Barron Associates, Inc, Blumio Inc, and Bold Diagnostics LLC among others.
Barron Associates, Inc. Company Overview: Barron Associates, Inc. (Barron) is a research and development company. It focuses on providing clients with novel solutions to demanding aerospace and healthcare challenges. The company provides products that include Adaptive Diagnostics and Prognostics Toolbox, Automated Pit Identification and Measurement Software, Fixed-Base Flight Simulator, and others. It also conducts services that include Human Interface Solutions, Signal/Data Processing & Analysis, Wearable Sensors for Physiologic Monitoring, and others. Barron is headquartered in Charlottesville, Virginia, the US.
Leading Blood Pressure Monitors Companies, 2023
For more company insights into the Blood Pressure Monitors pipeline market, download a free report sample
Blood Pressure Monitors Pipeline Market Segments
Blood Pressure Monitors Pipeline Market Segments Outlook
- Ambulatory Blood Pressure Monitors
- Wrist Blood Pressure Monitors
- Blood Pressure Transducers
- Upper Arm Blood Pressure Monitors
Blood Pressure Monitors Pipeline Market Territories Outlook
- China
- United States
- Global
- Europe
- Singapore
- Mexico
- Others
Blood Pressure Monitors Pipeline Market Regulatory Paths Outlook
- NMPA
- 510(k)
- CE
- HSA
- De novo
- MDITAC
- TGA
Scope
This report provides:
- Extensive coverage of the Blood Pressure Monitors under development.
- Details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Blood Pressure Monitors and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Blood Pressure Monitors under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
AtCor Medical Inc
Barron Associates, Inc.
Blumio Inc
Bold Diagnostics LLC
CardiacSense Ltd
Cardian Ltd
CardieX Ltd
CARDIOSIGN, INC.
Cardiostar Ltd.
Cirtec Medical Corp
CNSystems Medizintechnik AG
CoraVie Medical Inc
Department of Biomedical Engineering Columbia University
Digitouch Health LLC
Dynocardia Inc
DynoSense Corp.
Edwards Lifesciences Corp
Empirical Technologies Corporation
Huawei Technologies Co Ltd
Imperial College London
ImPress MedTech GmbH
Indian Institute of Technology Delhi
JB Healthtech Inc
Johns Hopkins University
Krisara Engineering, LLC
Lionsgate Technologies Inc.
Michigan State University
Monash University
Myant Inc
National University Health System Pte Ltd
Newcastle University
Northwestern University
Omron Healthcare Inc
Ovid BP Systems Inc
Pennsylvania State University
Pressao Medical
PyrAmes Inc
Qura Inc
Rice University
Sense A/S
Sensifree Inc
Shaare Zedek Medical Center
Shenzhen Shizhou Technology Co Ltd
Sonetics Ultrasound Inc
Sree Chitra Tirunal Institute for Medical Sciences & Technology
STBL Medical Research AG
SunTech Medical Inc
Texas A&M University
The Chinese University of Hong Kong
Tournicare Pty Ltd
Tufts University
University of California Los Angeles
University of California San Diego
University of Maryland Baltimore
University of Massachusetts
University of Michigan
University of Rochester
University of South Australia
Uscom Ltd
Valencell Inc
Vena Vitals Inc
Vmh Health, Incorporated
Wayne State University
Withings SA
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the Blood Pressure Monitors pipeline market?
Ambulatory blood pressure monitors is the leading segment in the Blood Pressure Monitors pipeline market.
-
Which territory has the highest number of pipeline products in the Blood Pressure Monitors pipeline market?
As of August 2023, the US has the highest number of pipeline products in the Blood Pressure Monitors pipeline market.
-
Which is the most followed regulatory pathway in the Blood Pressure Monitors pipeline market?
As of August 2023, 510(k) is the most followed regulatory pathway in the Blood Pressure Monitors pipeline market.
-
Who are the major players operating in the Blood Pressure Monitors pipeline market?
Some of the key companies in the Blood Pressure Monitors pipeline market are Aktiia SA, AtCor Medical Inc, Barron Associates, Inc, Blumio Inc, and Bold Diagnostics LLC among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Blood Pressure Monitors reports