Bioabsorbable Stents (BAS) – Pipeline Products by Stage of Development 13
Bioabsorbable Stents (BAS) – Pipeline Products by Territory 14
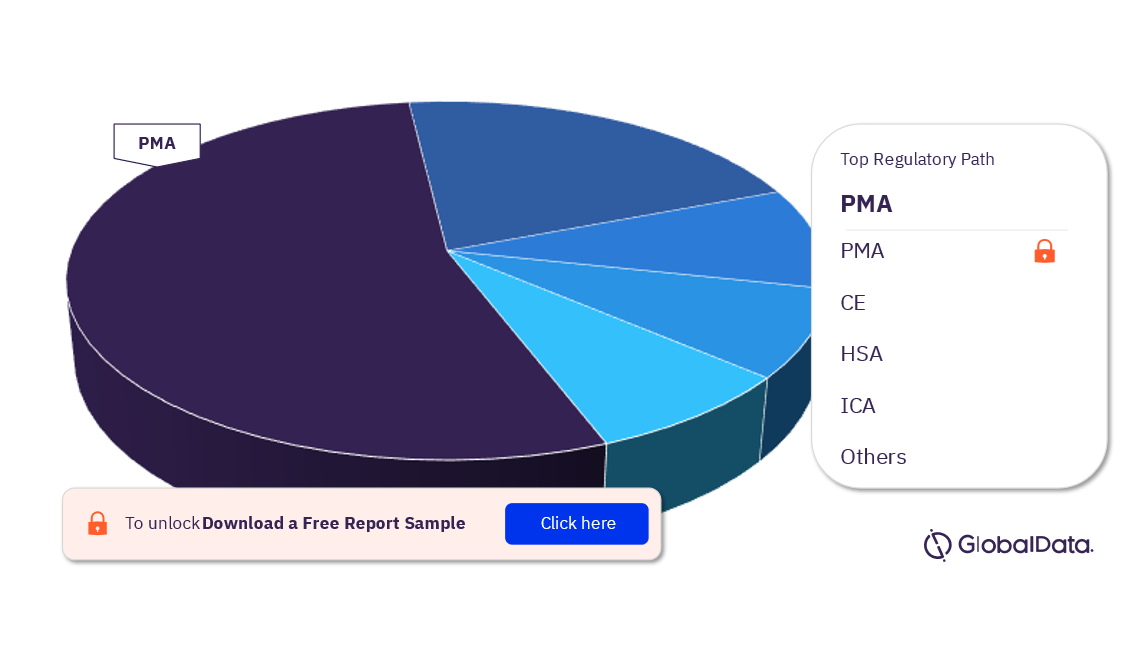
Bioabsorbable Stents (BAS) – Pipeline Products by Regulatory Path 15
Bioabsorbable Stents (BAS) – Pipeline Products by Estimated Approval Date 16
Bioabsorbable Stents (BAS) – Ongoing Clinical Trials 17
Bioabsorbable Stents (BAS) Companies – Pipeline Products by Stage of Development 18
Bioabsorbable Stents (BAS) – Pipeline Products by Stage of Development 20
Abbott Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 22
Second Generation Bioresorbable Vascular Scaffold – Product Status 22
Second Generation Bioresorbable Vascular Scaffold – Product Description 22
Amaranth Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 23
80-micron MAGNITUDE Bioresorbable Scaffold – Product Status 23
80-micron MAGNITUDE Bioresorbable Scaffold – Product Description 23
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Status 24
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Description 24
DEFIANCE Scaffold – Product Status 24
DEFIANCE Scaffold – Product Description 25
FORTITUDE Scaffold – Product Status 25
FORTITUDE Scaffold – Product Description 25
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Status 26
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Description 26
Second Generation FORTITUDE Scaffold – Product Status 26
Second Generation FORTITUDE Scaffold – Product Description 27
Amaranth Medical Inc – Ongoing Clinical Trials Overview 28
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Restoring Endoluminal Narrowing Using Bioresorbable Scaffolds – Extended Trial II 29
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Restoring Endoluminal Narrowing Using Bioresorbable Scaffolds – Extended Trial III 30
Arterius Ltd Pipeline Products & Ongoing Clinical Trials Overview 31
Arteriosorb Absorbable Drug-Eluting Scaffold – Product Status 31
Arteriosorb Absorbable Drug-Eluting Scaffold – Product Description 31
Biosensors International Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 32
Sparrow Drug Eluting Stent System – Product Status 32
Sparrow Drug Eluting Stent System – Product Description 32
Biosten, LLC Pipeline Products & Ongoing Clinical Trials Overview 33
Biodegradable Endovascular Implant – Product Status 33
Biodegradable Endovascular Implant – Product Description 33
Biotronik AG Pipeline Products & Ongoing Clinical Trials Overview 34
DREAMS 3G System – Product Status 34
DREAMS 3G System – Product Description 34
Magmaris Bioresorbable Magnesium Scaffold – Product Status 35
Magmaris Bioresorbable Magnesium Scaffold – Product Description 35
Biotronik AG – Ongoing Clinical Trials Overview 36
Magmaris Bioresorbable Magnesium Scaffold – A Sirolimus Eluting Bioresorbable Magnesium Stent for Treatment of Coronary Bifurcation Lesions: The BIFSORB Pilot Study II 37
Magmaris Bioresorbable Magnesium Scaffold – BIOTRONIK – Safety and Clinical Performance of the Drug Eluting Absorbable Metal Scaffold (DREAMS 2nd Generation) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries: BIOSOLVE-II 37
Magmaris Bioresorbable Magnesium Scaffold – Biotroniks – Safety and Performance in de Novo Lesion of Native Coronary Arteries with Magmaris- Registry: BIOSOLVE-IV 37
Magmaris Bioresorbable Magnesium Scaffold – Coronary Artery Healing Process after Bioresorbable Scaffold in Patients with Non-ST-segment Elevation Myocardial Infarction (NSTEMI) 38
Magmaris Bioresorbable Magnesium Scaffold – Optical Coherence Guided Treatment of ST-segment Elevation Myocardial Infarction with the Drug-eluting Resorbable Magnesium Scaffold: The BEST- MAG Multicentre Study (Belgian ST-segment Elevation Myocardial Infarction Treatment with Resorbable Magnesium Scaffold) 38
Magmaris Bioresorbable Magnesium Scaffold – Retrospective, Observational Register to Investigate the Procedural and Post Procedural Implantation of Bioabsorbable Magnesium Scaffolds Magmaris (MAGIC Registry) 38
Magmaris Bioresorbable Magnesium Scaffold – RMS (Resorbable Magnesium Scaffolds) Registry 39
Magmaris Bioresorbable Magnesium Scaffold – Scaffold Implantation in Emilia-Romagna 39
DREAMS 3G System – BIOTRONIK – Safety and Clinical Performance of the – Sirolimus-Eluting Resorbable Coronary Magnesium Scaffold System (DREAMS 3G) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries 40
DREAMS 3G System – BIOTRONIK – Safety and Clinical Performance of the Drug Eluting Resorbable Coronary Magnesium Scaffold System (DREAMS 3G) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries: BIOMAG-II: A Randomized Controlled Trial 40
Biotronik SE & Co KG Pipeline Products & Ongoing Clinical Trials Overview 41
AMS-2 – Product Status 41
AMS-2 – Product Description 41
Biotyx Medical (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 42
IBS Angel – Product Status 42
IBS Angel – Product Description 42
Biotyx Medical (Shenzhen) Co Ltd – Ongoing Clinical Trials Overview 43
IBS Angel – A Prospective, Multi-center, Single-arm Clinical Trial to Evaluate the Safety and Efficacy of Iron Bioresorbable Scaffold System (IBS Angel) in Patients with Pulmonary Artery Stenosis 44
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 45
Fully Resorbable Drug-Eluting Scaffold System – Product Status 45
Fully Resorbable Drug-Eluting Scaffold System – Product Description 46
Synergy Megatron Bioabsorbable Polymer (BP) Stent – Product Status 46
Synergy Megatron Bioabsorbable Polymer (BP) Stent – Product Description 46
Boston Scientific Corp – Ongoing Clinical Trials Overview 47
Synergy Megatron Bioabsorbable Polymer (BP) Stent – A U.S. Post-Approval Study of the SYNERGYTM Everolimus-Eluting Platinum Chromium Coronary Stent System Evaluating the SYNERGY XLV (MEGATRON) Stent System 48
Cardionovum GmbH Pipeline Products & Ongoing Clinical Trials Overview 49
Cardiosorb – Product Status 49
Cardiosorb – Product Description 49
Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview 50
SymBio Pimecrolimus/Paclitaxel-Eluting Stent – Product Status 50
SymBio Pimecrolimus/Paclitaxel-Eluting Stent – Product Description 50
HangZhou HuaAn Biotechnology Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview 51
Xinsorb BRS – Product Status 51
Xinsorb BRS – Product Description 51
Icon Interventional Systems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 52
Biosorb Pediatric Dissolving Stent – Product Status 52
Biosorb Pediatric Dissolving Stent – Product Description 52
Japan Stent Technology Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview 53
Bioabsorbable Stent – Product Status 53
Bioabsorbable Stent – Product Description 53
Kyoto Medical Planning Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 54
Igaki-Tamai Coronary Stent – Product Status 54
Igaki-Tamai Coronary Stent – Product Description 54
Lifetech Scientific (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 55
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Product Status 55
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Product Description 55
Lifetech Scientific (Shenzhen) Co Ltd – Ongoing Clinical Trials Overview 56
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A First-in-man Study of Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System (IBS) 57
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Multi-center, Single-arm Trial of the Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System in Patients with Coronary Artery Disease: IRONMAN-III 57
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Multi-center, Single-blinded, Randomized Trial of the Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System in Patients with Coronary Artery Disease: IRONMAN-II 57
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Non-randomized, Open-label, Non-comparative, First-in-Man Study to Evaluate the Feasibility and Safety of Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System 58
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Feasibility, Efficacy and Safety of IBS for Implantation in the PDA in Duct-dependent Cyanotic CHD 58
Manli Cardiology Ltd Pipeline Products & Ongoing Clinical Trials Overview 59
Mirage – Product Status 59
Mirage – Product Description 59
Meril Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 60
MeRes100 – Product Status 60
MeRes100 – Product Description 60
Meriskin BRS – Product Status 61
Meriskin BRS – Product Description 61
Meril Life Sciences Pvt Ltd – Ongoing Clinical Trials Overview 62
MeRes100 – A Multi Center Randomized Control Study of MeRes100 Sirolimus Eluting BioResorbable Vascular Scaffold System in Treatment of Coronary Artery Disease Patients: MeRes – China 63
MeRes100 – A Prospective, Multinational, Multicenter, Single Arm, Open Label, Pilot Clinical Study of MeRes100 Sirolimus Eluting Bioresorbable Vascular Scaffold System in the Treatment of De-novo Native Coronary Artery Lesions: MeRes-1 Extend 63
MeRes100 – To Compare the Safety and Performance of MeRes100 Sirolimus Eluting BioResorbable Vascular Scaffold System Versus Contemporary DES Platforms in Patients With de Novo Coronary Artery Lesions 63
Micell Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 64
Bioresorbable Scaffold (BRS) – Product Status 64
Bioresorbable Scaffold (BRS) – Product Description 64
Michigan Technological University Pipeline Products & Ongoing Clinical Trials Overview 65
Bioabsorbable Stent – Product Status 65
Bioabsorbable Stent – Product Description 65
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 66
Firefalcon Bioresorbable Device – Product Status 66
Firefalcon Bioresorbable Device – Product Description 66
Firesorb Bioresorbable Scaffold – Product Status 67
Firesorb Bioresorbable Scaffold – Product Description 67
MicroPort Scientific Corp – Ongoing Clinical Trials Overview 68
Firesorb Bioresorbable Scaffold – A First-in-man Study of the Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold in Patients with Coronary Artery Disease: FUTURE-I 69
Firesorb Bioresorbable Scaffold – A Prospective Multicenter Single-arm Clinical Trial Assessing the Safety and Effectiveness of Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold for the Treatment of Coronary Artery Disease: FUTURE III 69
Firesorb Bioresorbable Scaffold – A Randomized Trial of the Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold in Patients with Coronary Artery Disease: FUTURE-II 69
Minvasys SAS Pipeline Products & Ongoing Clinical Trials Overview 70
Nile LM SIR Sirolimus Eluting Intracoronary Stent – Product Status 70
Nile LM SIR Sirolimus Eluting Intracoronary Stent – Product Description 70
MIV Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 71
Smart-1 DES – Product Status 71
Smart-1 DES – Product Description 71
Smart-4 DES – Product Status 72
Smart-4 DES – Product Description 72
VESTAsync – Product Status 72
VESTAsync – Product Description 73
NanoCoeur, Inc. Pipeline Products & Ongoing Clinical Trials Overview 74
Nanocoating Cardiac Stent – Product Status 74
Nanocoating Cardiac Stent – Product Description 74
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 75
Biodegradable Metal Stent – Product Status 75
Biodegradable Metal Stent – Product Description 75
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 76
Liquid Cast Drug-Eluting Biodegradable Stent – Product Status 76
Liquid Cast Drug-Eluting Biodegradable Stent – Product Description 76
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 77
Cura Stent – Product Status 77
Cura Stent – Product Description 77
Sirolimus-Eluting Absorbable Vascular Scaffold – Product Status 78
Sirolimus-Eluting Absorbable Vascular Scaffold – Product Description 78
Pediastent LLC Pipeline Products & Ongoing Clinical Trials Overview 79
Pediatric Bioresorbable Stent – Product Status 79
Pediatric Bioresorbable Stent – Product Description 79
QualiMed Innovative Medizinprodukte GmbH Pipeline Products & Ongoing Clinical Trials Overview 80
Biodegradable Coronary Stent – Product Status 80
Biodegradable Coronary Stent – Product Description 80
REVA Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 81
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Status 81
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Description 82
REVA Medical Inc – Ongoing Clinical Trials Overview 83
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Expanded FANTOM II Clinical Study 84
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Post Market Study of the FANTOM Sirolimus-eluting Bioresorbable Coronary Scaffold 84
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Safety and Efficacy of the FANTOM ENCORE Sirolimus-eluting Bioresorbable Scaffold for Treatment of De-novo Coronary Artery Disease: The ENCORE-I Study 84
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Safety and Performance Study of the FANTOM Sirolimus-eluting Bioresorbable Coronary Scaffold 85
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Vascular Healing Pattern, Vasoreactivity, and Quality of Life in Patients with ST Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention with Sirolimus Eluting FANTOM Bioresorbable Vascular Scaffold with Long Term Clinical, Near Infrared Spectroscopy and Optical Coherence Tomography Follow-up: A FANTOM STEMI Pilot Study 85
S3V Vascular Technologies Pipeline Products & Ongoing Clinical Trials Overview 86
3V Avatar – Product Status 86
3V Avatar – Product Description 86
Shanghai Bio-heart Biological Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
Bioheart BRS System – Product Status 87
Bioheart BRS System – Product Description 87
Bioheart Ultra BRS System – Product Status 88
Bioheart Ultra BRS System – Product Description 88
Shanghai Bio-heart Biological Technology Co Ltd – Ongoing Clinical Trials Overview 89
Bioheart BRS System – A Randomized Controlled Trial of the Bioheart Rapamycin Drug-Eluting Bioresorbable Coronary Stent System in Patients With Coronary Artery Disease: BIOHEART-II 90
Bioheart BRS System – A Registry Trial of the Bioheart Rapamycin Drug-eluting Bioresorbable Coronary Stent System in Patients with Coronary Artery Disease: BIOHEART III 90
Bioheart BRS System – Study of Bioheart BRS System for the Treatment of Coronary Artery Disease 90
Shanghai Healing Medical Instruments Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 91
Biodegradable Sinus Stent – Product Status 91
Biodegradable Sinus Stent – Product Description 91
Tepha Inc Pipeline Products & Ongoing Clinical Trials Overview 92
TephaFLEX Absorbable Coronary Stent – Product Status 92
TephaFLEX Absorbable Coronary Stent – Product Description 92
Tremedics Medical Devices LLC Pipeline Products & Ongoing Clinical Trials Overview 93
Illusicor – Product Status 93
Illusicor – Product Description 93
UAB Minority Health and Health Equity Research Center Pipeline Products & Ongoing Clinical Trials Overview 94
Endothelium Nanomatrix Coated Stent – Product Status 94
Endothelium Nanomatrix Coated Stent – Product Description 94
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 95
Nanocomposite Drug Eluting Stent – Product Status 95
Nanocomposite Drug Eluting Stent – Product Description 95
University of Strathclyde Pipeline Products & Ongoing Clinical Trials Overview 96
Magnesium Alloy Stent – Product Status 96
Magnesium Alloy Stent – Product Description 96
VasoTech Inc. Pipeline Products & Ongoing Clinical Trials Overview 97
PowerStent Absorb DES – Product Status 97
PowerStent Absorb DES – Product Description 97
Xenogenics Corporation Pipeline Products & Ongoing Clinical Trials Overview 98
Ideal BioStent – First Generation – Product Status 98
Ideal BioStent – First Generation – Product Description 98
Ideal BioStent – Second Generation – Product Status 99
Ideal BioStent – Second Generation – Product Description 99
Whisper Coronary Stent System – Product Status 99
Whisper Coronary Stent System – Product Description 100
Glossary 110
![]()
 For more territory insights into the BAS pipeline products market, download a free report sample
For more territory insights into the BAS pipeline products market, download a free report sample