Anesthesia Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2021
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Anesthesia Devices are used to administer anesthesia during surgery. Anesthesia Devices includes anesthesia machines, anesthesia circuits, breathing filters, breathing bags, anesthesia masks and regional anesthesia disposables. An anesthesia machine is used for administration of anesthetic gases, vapors and for controlling ventilation throughout the anesthetic procedure. It has evolved from a simple pneumatic device to a complex array of mechanical, electrical and computer-controlled components. An anesthesia machine consists of oxygen (O2) flow meter, ventilator, vaporizer (which produces vapor from a volatile liquid anesthetic agent), breathing system, absorber system, source of oxygen and a scavenging device that removes any excess anesthetic gases present in the operating room to maintain clinical hygiene.
Anesthetic agents such as isoflurane, halothane which are used in an anesthesia machine are not tracked in this segment. An anesthesia workstation is the advanced workstation with anesthesia delivery, respiratory gas delivery and monitoring system. A portable anesthesia delivery machine is lightweight and easy to transport. It is comparatively lower in configuration and compact enough for the smallest surgery or imaging suite.
What are the market dynamics in the anesthesia devices pipeline products market?
As of December 2021, 4 pipeline product was in the approval process, 6 pipeline products were in the inactive stage and 4 pipeline products were in the early stage of development. 7 pipeline products were in the clinical stage and 1 was in the indeterminate stage. The anesthesia machines accounted for the largest share of more than 25% in the segments of anesthesia device pipeline products followed by epidural needles, regional anesthesia disposables, spinal needles, and portable anesthesia delivery machines. The US is leading the pipeline products followed by Europe, China, Australia, Vietnam, India, Japan, South Korea, and the UK.
Which are the key players in the anesthesia devices pipeline products market?
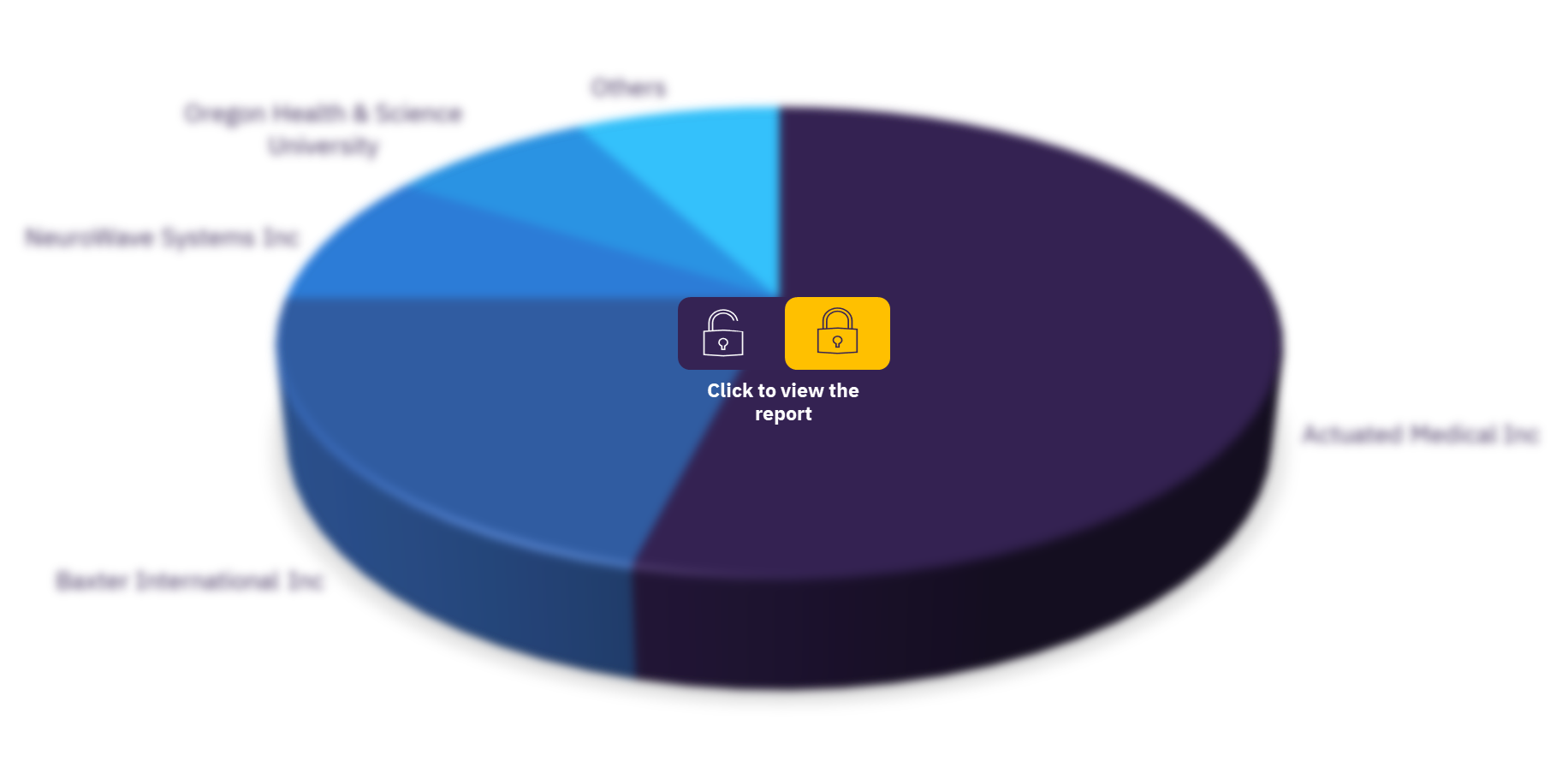
The key players in the market are Actuated Medical Inc, Ancora Medical Technology, Baxter International Inc, DOME Medical Technologies Inc, EP Global Communications Inc (Inactive), First Wave Technologies Inc, Flat Medical Inc, Injeq Oy, Medovate Ltd, NeuroWave Systems Inc, Omeq Medical Ltd., Oregon Health & Science University, Recens Medical Co Ltd, Stela Medical LLC, University of Michigan, University of Nebraska, and Virginia Commonwealth University.
Company overview:
Actuated Medical Inc: Actuated Medical Inc (Actuated Medical) is a medical device company that develops minimally invasive instruments. The company provides products such as controlled tissue penetration systems, occlusion clearing systems and MRI compatible systems. Its controlled tissue penetration systems use low force, electronically controlled motion to enable smooth insertion of sharps for serial blood sampling procedures. Actuated Medical’s also provides tube clearing solutions that remove clogs in patient. The company’s occlusion clearing systems use mechanical motion to restore patency of tubes in the patient for clearing clogged nasoenteral, gastrostomy, nasogastric, and jejunostomy feeding and decompression tubes. It operates through manufacturing facility in Bellefonte. Actuated Medical is headquartered in Bellefonte, Pennsylvania, the US. The company manufactured the Low Insertion Force Epidural System (LIFE) is a spinal anesthesia disposable device intended for anesthesia delivery. It is designed to provide controlled epidural needle entry into the epidural space for regional anesthesia.
Baxter International Inc: Baxter International Inc (Baxter) is a provider of healthcare products, including dialysis in acute and chronic conditions, sterile intravenous solutions, generic injectable pharmaceuticals, parenteral nutrition therapies, infusion systems and devices, inhaled anaesthetics, and surgical hemostat and sealant products. The company’s products find application in hospitals, rehabilitation centers, kidney dialysis centers, nursing homes, besides patients at home under physician supervision. Baxter distributes products through direct sales force and a network of independent distributors, drug wholesalers, specialty pharmacies and other alternative site providers. The company has operations in the Americas, the Middle East, Africa, Europe and Asia-Pacific. Baxter is headquartered in Deerfield, Illinois, the US. The company manufactured the Ecocapture that is an anesthetic gas filter intended for anaesthesia delivery. It is designed to provide physiological levels of humidification to the patient’s breathing gases.
NeuroWave Systems Inc: NeuroWave Systems Inc (NeuroWave) is a medical device company that develops and commercializes brain monitors with electrodes. The company offers products such as neurosense monitor and easyprep electrodes. Its NeuroSENSE monitor is a bilateral brain monitor, which measure anesthetic effects on the brain and respond instantaneously to changes in patient state. NeuroWave’s EasyPrep electrodes is a proprietary disposable EEG electrode, which are designed for NeuroWave’s brain monitoring technologies. The company provides services such as advanced signal processing technologies, medical software, and electrophysiological device development and safety, EMC, risk assessment, and software. It offers products and services in the research areas of peri-operative neuromonitoring, seizure detection and sleep architecture assessment. The company markets its products through a network of distributors in Austria, Belgium, Canada, Luxembourg, Montenegro, the Netherlands and Serbia. NeuroWave is headquartered in Cleveland Heights, Ohio, the US.
The company manufactured the AutoSED is an infusion intravenous sedation delivery system intended for anesthesia delivery in forward surgical care (FSC) for stabilized casualties who are awaiting medical evacuation to hospital. It is designed to deliver intravenous sedation analgesia and fluids via infusion pumps using rotary peristaltic mechanism with epicyclical gears.
Oregon Health & Science University: Oregon Health & Science University (OHSU) is an academic health organization that provides medical education, research, patient care and community services. The organization offers undergraduate and graduate courses in anesthesiology, dermatology, neurology, psychiatry, periodontology and nursing through its educational institute. It also grants doctoral degrees in medical physics, clinical psychology, neuroscience, biomedical engineering and other areas. OHSU provides healthcare services such as bone and joint care, cancer care, heart care, neuroscience, pediatrics, spine care and women’s health. The organization conducts research in disease areas including cancer, rare genetic disorders, diseases of the central nervous system, cardiovascular-related research and infectious disease. It has locations across Oregon and South West Washington. OHSU is headquartered in Portland, Oregon, the US.
University of Michigan: University of Michigan (UM) is an educational service provider that offers educational and research services. The university offers graduate, undergraduate and post-doctoral levels programs in various disciplines. It operates various graduate schools and colleges of graduate studies, architecture, urban planning, education, engineering, information, kinesiology, law, medicine, music, theatre, dance, natural resources, environment, nursing and pharmacy. The university offers undergraduate studies in the areas of literature, science, architecture, urban, planning, art, design, business, education nursing, pharmacy and public policy. It also provides digital, lifelong and summer courses and programs. UM is headquartered in Ann Arbor, Michigan, the US.
University of Nebraska: University of Nebraska is an educational institution that provides graduate, undergraduate and postgraduate courses. The institute offers undergraduate and postgraduate degrees in the areas of business, agriculture, engineering, medicine, and others. It provides research areas such as nanotechnology and alternative energy, transportation and highway safety, agriculture and natural resources, early childhood education, and others. The University of Nebraska provides research in the areas of cancer, organ transplantation, diabetes, aging, undergraduate creative activities and research experiences programheuetmann lectures focus on the sustainability of food, renewable energy, natural resources and rural communities neurodegenerative diseases and a wide range of public health issues, among others. The institute operates through its campuses located in Curtis, Kearney, Nebraska, Lincoln and Omaha, among others. University of Nebraska is headquartered in Lincoln, Nebraska, the US.
Anesthesia devices pipeline products, by key players
To know more about key players, download a free report sample
Market report scope
| Key countries/regions | The US, Europe, China, Australia, Vietnam, India, Japan, South Korea, and the UK. |
| Key players | Actuated Medical Inc, Ancora Medical Technology, Baxter International Inc, DOME Medical Technologies Inc, EP Global Communications Inc (Inactive), First Wave Technologies Inc, Flat Medical Inc, Injeq Oy, Medovate Ltd, NeuroWave Systems Inc, Omeq Medical Ltd., Oregon Health & Science University, Recens Medical Co Ltd, Stela Medical LLC, University of Michigan, University of Nebraska, and Virginia Commonwealth University |
Scope
- Extensive coverage of the Anesthesia Devices under development.
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities.
- The report reviews the major players involved in the development of Anesthesia Devices and list all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from Early Development to the Approved/Issued stage.
- The report provides key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of Anesthesia Devices under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Ancora Medical Technology
Baxter International Inc
DOME Medical Technologies Inc
EP Global Communications Inc (Inactive)
First Wave Technologies Inc
Flat Medical Inc
Injeq Oy
Medovate Ltd
NeuroWave Systems Inc
Omeq Medical Ltd.
Oregon Health & Science University
Recens Medical Co Ltd
Stela Medical LLC
University of Michigan
University of Nebraska
Virginia Commonwealth University
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key geographic regions in the anesthesia devices pipeline products market?
The US is leading the pipeline products followed by Europe, China, Australia, Vietnam, India, Japan, South Korea, and the UK.
-
Who are the key players in the anesthesia devices pipeline products market?
The key players in the market are Actuated Medical Inc, Ancora Medical Technology, Baxter International Inc, DOME Medical Technologies Inc, EP Global Communications Inc (Inactive), First Wave Technologies Inc, Flat Medical Inc, Injeq Oy, Medovate Ltd, NeuroWave Systems Inc, Omeq Medical Ltd., Oregon Health & Science University, Recens Medical Co Ltd, Stela Medical LLC, University of Michigan, University of Nebraska, and Virginia Commonwealth University.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Anesthesia and Respiratory Devices reports